基于取代的钡铈锆酸盐的三导电多组分氧化物的热电和电学特性
IF 3.5
3区 化学
Q2 CHEMISTRY, INORGANIC & NUCLEAR
引用次数: 0
摘要
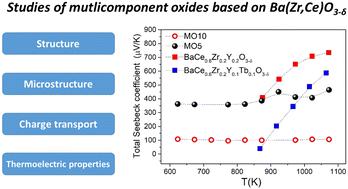
多组分氧化物通常具有优异的热稳定性和有趣的电子特性。本研究介绍了 Ba(Zr0.2Hf0.2Sn0.2Ti0.2Fe0.2)O3-δ 和 Ba(Zr0.1Hf0.1Sn0.1Ti0.1Co0.1Ce0.1Bi0.1Fe0.1Y0.1Zn0.1)O3-δ 多组分包晶的热电和电学特性。采用固态反应方法合成了单相立方包晶。使用 X 射线衍射、滴溶量热和热重法对它们进行了表征。研究发现,Ba(Zr0.1Hf0.1Sn0.1Ti0.1Co0.1Ce0.1Bi0.1Fe0.1Y0.1Zn0.1)O3-δ 的热力学稳定性低于 Ba(Zr0.2Hf0.2Sn0.2Ti0.2Fe0.2)O3-δ。此外,这种氧化物含有较多的水,具有较高的导电性和较低的塞贝克系数。这两种过氧化物中的电荷传输都可以归结为通过电子空穴进行的小极子跳跃过程。研究发现,塞贝克系数具有有趣的温度依赖性,在 750 K 以上的温度下,塞贝克系数与能量不等态间的跳变有关。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Thermoelectric and electrical properties of triple-conducting multicomponent oxides based on substituted barium cerate-zirconate
Multicomponent oxides often have exceptional thermal stability and interesting electronic properties. The present work presents the thermoelectric and electrical properties of the Ba(Zr0.2Hf0.2Sn0.2Ti0.2Fe0.2)O3−δ and Ba(Zr0.1Hf0.1Sn0.1Ti0.1Co0.1Ce0.1Bi0.1Fe0.1Y0.1Zn0.1)O3−δ multicomponent perovskites. Single-phase cubic perovskites were synthesized using the solid-state reaction method. They were characterized using X-ray diffraction, drop-solution calorimetry, and thermogravimetry methods. The total electrical conductivity and Seebeck coefficient measurements were performed in dry and wet air at temperatures between 600 and 1050 K. It was found that Ba(Zr0.1Hf0.1Sn0.1Ti0.1Co0.1Ce0.1Bi0.1Fe0.1Y0.1Zn0.1)O3−δ is thermodynamically less stable than Ba(Zr0.2Hf0.2Sn0.2Ti0.2Fe0.2)O3−δ. Moreover, this oxide incorporates a higher amount of water and exhibits higher conductivity and lower Seebeck coefficient. Charge transport in both perovskites can be assigned to the small-polaron hopping process via electron holes. An interesting temperature dependence of the Seebeck coefficient was found and, at temperatures above 750 K, related to hopping between energetically inequivalent states.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Dalton Transactions
化学-无机化学与核化学
CiteScore
6.60
自引率
7.50%
发文量
1832
审稿时长
1.5 months
期刊介绍:
Dalton Transactions is a journal for all areas of inorganic chemistry, which encompasses the organometallic, bioinorganic and materials chemistry of the elements, with applications including synthesis, catalysis, energy conversion/storage, electrical devices and medicine. Dalton Transactions welcomes high-quality, original submissions in all of these areas and more, where the advancement of knowledge in inorganic chemistry is significant.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


