在电化学处理镀镍废水过程中,碳毡阴极上形成的 Ni(0) 触发了 Ni-EDTA 的氧化还原协同降解作用
IF 11.3
1区 环境科学与生态学
Q1 ENGINEERING, ENVIRONMENTAL
引用次数: 0
摘要
镍有机配合物广泛应用于工业生产,对自然环境和人类健康构成严重威胁。在本研究中,我们发现典型的Ni有机配合物(Ni- edta)在碳毡阴极表面分解还原形成Ni(0)。形成的Ni(0)作为催化位点,通过电子转移生成具有强还原活性的原子氢(H•)。H•在碳毡阴极上加速氧活化生成H2O2,并与H2O2反应生成强氧化羟基自由基(HO•)和单线态氧(1O2)。在−2.0 V (vs. Ag/AgCl)条件下,电解120 min后,Ni- edta的去除率达到95.5 %,总有机碳(TOC)和溶解Ni的去除率分别达到43.8% %和77.1 %。碳毡阴极上Ni(0)的生成引发氧化还原协同反应,在处理真实镀镍废水中表现出优异的稳定性。这种方法不需要额外的化学试剂,并防止重金属的释放。我们的研究结果为利用Ni有机配合物的自转化来产生各种活性物质用于镀镍废水的电化学处理提供了一种新的策略。本文章由计算机程序翻译,如有差异,请以英文原文为准。
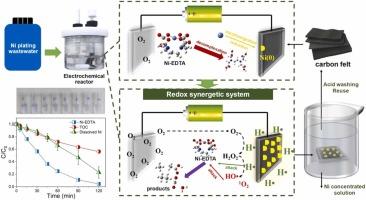
Ni(0) formation on carbon felt cathode triggers redox synergetic degradation of Ni-EDTA during electrochemical treatment for Ni-plating wastewater
Ni organic complexes are widely used in industrial production, posing severe threats to the natural environment and human health. In this work, we found that Ni (0) formed by the decomplexation and reduction of typical Ni organic complexes (Ni-EDTA) on the surface of carbon felt cathode. The formed Ni(0) as a catalytic site generates atomic hydrogen (H•) with strong reductive reactivity via electron transfer. H• not only accelerates the oxygen activation to form H2O2 on carbon felt cathode, but also reacts with H2O2 to generate strong oxidative hydroxyl radicals (HO•) and singlet oxygen (1O2). Under the attack of multiple active species, 95.5 % of Ni-EDTA is removed, and the removal efficiencies of total organic carbon (TOC) and dissolved Ni reach 43.8 % and 77.1 % after 120 min of electrolysis at −2.0 V (vs. Ag/AgCl). The redox synergistic reaction, initiated by the formation of Ni(0) on the carbon felt cathode, demonstrates excellent stability in treating real Ni plating wastewater. This approach eliminates the need for additional chemical reagents and prevents the release of heavy metals. Our findings offer a novel strategy for leveraging the self-transformation of Ni organic complexes to generate various active species for electrochemical treatment of Ni plating wastewater.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Hazardous Materials
工程技术-工程:环境
CiteScore
25.40
自引率
5.90%
发文量
3059
审稿时长
58 days
期刊介绍:
The Journal of Hazardous Materials serves as a global platform for promoting cutting-edge research in the field of Environmental Science and Engineering. Our publication features a wide range of articles, including full-length research papers, review articles, and perspectives, with the aim of enhancing our understanding of the dangers and risks associated with various materials concerning public health and the environment. It is important to note that the term "environmental contaminants" refers specifically to substances that pose hazardous effects through contamination, while excluding those that do not have such impacts on the environment or human health. Moreover, we emphasize the distinction between wastes and hazardous materials in order to provide further clarity on the scope of the journal. We have a keen interest in exploring specific compounds and microbial agents that have adverse effects on the environment.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


