IF 13
2区 材料科学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
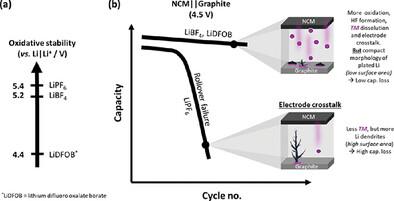
提高上截止电压(UCV)可增强锂离子电池(LIB)的比能量,但同时也会因电极串扰(即过渡金属(TM)从阴极溶出并沉积在阳极上,最终因局部电阻增强而引发高比表面积锂(HSAL)的形成)而导致更高的容量衰减。本文研究了碳酸盐溶剂中的 LiPF6、LiBF4、二氟(草酸盐)硼酸锂(LiDFOB)、双(草酸盐)硼酸锂(LiBOB)、双(氟磺酰)亚胺锂(LiFSI)和双(三氟甲磺酰)亚胺锂(LiTFSI)在 LiNi0.6Co0.2Mn0.2O2 (NCM 622) || 4.5 V UCV 的石墨袋电池中进行了研究。尽管 LiBF4 和 LiDFOB 的氧化稳定性较低,从而增强了 HF 的形成、TM 的溶解以及由此产生的电极串扰,但与 LiPF6 电解液相比,观察到了更高的容量保持率。与直觉相反的是,影响容量衰减的不是 TM 沉积量,而是锂镀层的形态,因为这些盐会使锂镀层更均匀、更紧凑,即表面积更小。相比之下,含 LiPF6 的树枝状 HSAL 表面积更大,寄生反应更多,因此活性锂("锂库存")损失和容量衰减也更大。虽然 NCM 引发了失效级联,但高压 LIB 的容量损失和循环寿命主要取决于阳极,特别是锂镀层形态和相应的表面积。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Evaluation of Alternative Lithium Salts for High-Voltage Lithium Ion Batteries: Higher Relevance of Plated Li Morphology Than the Amount of Electrode Crosstalk
Increasing the upper cut-off voltage (UCV) enhances the specific energy of Li-ion batteries (LIBs), but is accompanied by higher capacity fade as a result of electrode cross-talk, i.e., transition metals (TM) dissolution from cathode and deposition on anode, finally triggering high surface area lithium (HSAL) formation due to locally enhanced resistance. Here, LiPF6, LiBF4, lithium difluoro(oxalate)borate (LiDFOB), lithium bis(oxalate)borate (LiBOB), lithium bis(fluorosulfonyl)imide (LiFSI), and lithium bis(trifluoromethanesulfonyl)imide (LiTFSI) in carbonate-based solvents are investigated in LiNi0.6Co0.2Mn0.2O2 (NCM 622) || graphite pouch cells with 4.5 V UCV. Despite the lower oxidative stabilities of LiBF4 and LiDFOB, thus enhanced HF formation, TM dissolution, and consequently electrode cross-talk, higher capacity retention is observed compared to the case of LiPF6 electrolyte. Counterintuitively, it is not the TM deposit amount but rather the Li plating morphology that governs capacity fade, as these salts cause more uniform and compact lithium plating, i.e., lower surface area. In contrast, the dendritic HSAL with LiPF6 has a higher surface area, and more parasitic reactions, thus active Li (“Li inventory”) losses and capacity fade. Although NCM initiates the failure cascade, the capacity losses and cycle life of high-voltage LIBs are predominantly determined by the anode, in particular the Li plating morphology and the corresponding surface area.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Small
工程技术-材料科学:综合
CiteScore
17.70
自引率
3.80%
发文量
1830
审稿时长
2.1 months
期刊介绍:
Small serves as an exceptional platform for both experimental and theoretical studies in fundamental and applied interdisciplinary research at the nano- and microscale. The journal offers a compelling mix of peer-reviewed Research Articles, Reviews, Perspectives, and Comments.
With a remarkable 2022 Journal Impact Factor of 13.3 (Journal Citation Reports from Clarivate Analytics, 2023), Small remains among the top multidisciplinary journals, covering a wide range of topics at the interface of materials science, chemistry, physics, engineering, medicine, and biology.
Small's readership includes biochemists, biologists, biomedical scientists, chemists, engineers, information technologists, materials scientists, physicists, and theoreticians alike.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


