土壤有机质成分在与矿物质相互作用过程中的协同效应
IF 11.3
1区 环境科学与生态学
Q1 ENGINEERING, ENVIRONMENTAL
引用次数: 0
摘要
与矿物相关的土壤有机质(SOM)对于稳定有机碳和减缓气候变化至关重要。然而,人们对矿物-SOM 在分子尺度上的相互作用,尤其是通过矿物表面有机-有机相互作用(即有机多层化)产生的协同吸附作用,仍然知之甚少。本研究通过整合宏观尺度实验和分子尺度模拟,评估土壤矿物上主要 SOM 化合物--月桂酸(脂质)、五甘氨酸(氨基酸)、三卤糖(碳水化合物)和木质素--的单独和顺序吸附,从而研究有机多层化对矿物-SOM 相互作用的影响。将铁酸盐、氢氧化铝和方解石与 SOM 化合物接触,以确定吸附亲和力和结合能。结果表明,月桂酸的 Kd 值是五甘氨酸的 20-40 倍,其顺序为 Kd(铁酸盐)> Kd(氢氧化铝)≫Kd(方解石)。分子尺度模拟证实,月桂酸在铁水物上的结合能(30.8 kcal/mol)高于五甘氨酸(6.0 kcal/mol),这归因于脂质的疏水性。五甘氨酸的结合能较低是由于它的亲水酰胺基团促进了与水的分离。连续实验研究了第一层脂质或氨基酸如何影响碳水化合物/木质素的吸附,这两种物质几乎没有单独的吸附亲和力。宏观实验结果表明,脂质和氨基酸的吸附会引起亚铁酸盐颗粒斥力,增加反应表面积,并通过有机多层化作用独立和协同地增强对碳水化合物/木质素的吸附。分子尺度结果显示,吸附在亚铁酸盐上的氨基酸更容易与木质素大聚合体(在溶液中预先形成)发生作用,而不是与单个木质素单元发生作用,这表明有机物通过 H 键发生了多层化作用。这些发现揭示了 SOM 与矿物质相互作用的分子机制,对提高土壤碳稳定性至关重要。本文章由计算机程序翻译,如有差异,请以英文原文为准。
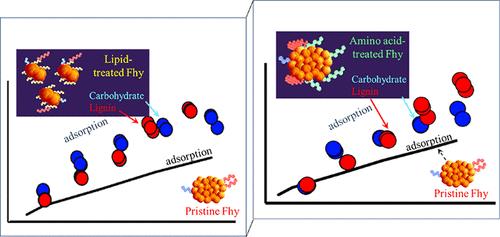
Synergetic Effects of Soil Organic Matter Components During Interactions with Minerals
Mineral-associated soil organic matter (SOM) is critical for stabilizing organic carbon and mitigating climate change. However, mineral-SOM interactions at the molecular scale, particularly synergetic adsorption through organic–organic interaction on the mineral surface known as organic multilayering, remain poorly understood. This study investigates the impact of organic multilayering on mineral-SOM interactions, by integrating macroscale experiments and molecular-scale simulations that assess the individual and sequential adsorption of major SOM compounds–lauric acid (lipid), pentaglycine (amino acid), trehalose (carbohydrate), and lignin onto soil minerals. Ferrihydrite, Al-hydroxide, and calcite are exposed to SOM compounds to determine adsorption affinities and binding energies. Results show that lauric acid has 20–40 times higher Kd than pentaglycine, following the order Kd(ferrihydrite) > Kd(Al-hydroxide) ≫ Kd(calcite). Molecular-scale simulations confirm that lauric acid has a higher binding energy (30.8 kcal/mol) on ferrihydrite than pentaglycine (6.0 kcal/mol), attributed to lipid hydrophobicity. The lower binding energy of pentaglycine results from its hydrophilic amide groups, facilitating partitioning into water. Sequential experiments examine how the first layer of lipid or amino acid affects the adsorption of carbohydrate/lignin, which show little or no individual adsorption affinities. Macroscale results reveal that lipid and amino acid adsorption induce ferrihydrite particle repulsion increasing reactive surface area and enhancing carbohydrate/lignin adsorption independently and synergistically through organic multilayering. Molecular-scale results reveal that amino acid adsorbed on ferrihydrite interacts more readily with lignin macroaggregates (preformed in solution) than with individual lignin units, indicating organic multilayering via H-bonding. These findings reveal the molecular mechanisms of SOM-mineral interactions, crucial for enhancing soil carbon stabilization.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

环境科学与技术
环境科学-工程:环境
CiteScore
17.50
自引率
9.60%
发文量
12359
审稿时长
2.8 months
期刊介绍:
Environmental Science & Technology (ES&T) is a co-sponsored academic and technical magazine by the Hubei Provincial Environmental Protection Bureau and the Hubei Provincial Academy of Environmental Sciences.
Environmental Science & Technology (ES&T) holds the status of Chinese core journals, scientific papers source journals of China, Chinese Science Citation Database source journals, and Chinese Academic Journal Comprehensive Evaluation Database source journals. This publication focuses on the academic field of environmental protection, featuring articles related to environmental protection and technical advancements.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


