电化学剥离钾盐中的二氧化碳,促进直接空气捕获
IF 5.6
3区 材料科学
Q1 ELECTROCHEMISTRY
引用次数: 0
摘要
这项工作介绍了一种电化学再生器的实验研究,该再生器用于从碳酸钾中剥离二氧化碳并回收氢氧化钾。本文章由计算机程序翻译,如有差异,请以英文原文为准。
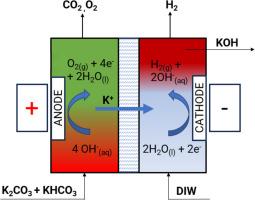
Electrochemical stripping of CO2 from potassium-based salts to facilitate direct air capture
This work presents an experimental study of an electrochemical regenerator for stripping CO2 and recovering potassium hydroxide from potassium carbonate/bicarbonate streams. This regenerator is applicable in the regenerative step of an aqueous Direct Air Capture (DAC) process during which CO2 is captured from air using potassium hydroxide. Experimental tests were conducted in a near zero-gap cell designed with nickel foam electrodes to show CO2 stripping from carbon-loaded streams, investigating the effects of current, flowrate, and concentration of anolyte and catholyte, on the performance of the electrochemical regenerator in terms of the CO2 stripping efficiency (CSE) and energy consumption (EC). Results from the tests show that about 90 percent of the CSE is achievable in this process with a mixture of 0.15 M potassium carbonate and 0.15 M potassium bicarbonate as the feed stream at potassium factor, of ∼1, whereby decreasing the alkalinity to carbon ratio can further boost the CSE under the same current density. The study shows that increasing the potassium factor favors the CSE, while increasing the buffering factor has a negative impact on the CSE. Furthermore, for potassium factors below the “buffering threshold”, the CSE is negligible. Results also show that the CSE is constant for varying applied current at constant potassium factor. In addition, the influence of supporting electrolyte (SEL) in the cathode chamber of the regenerator, on both the CSE and the voltage penalty is studied. The use of concentrated KOH in the cathode helps to reduce voltage penalty. However, it shows a detrimental effect on the CSE due to the uphill difference in K+ concentration between the two chambers, compared to deionized water. Experimental results show energy consumption in the range of 250–500 kJ/mol-CO2 as a lower range of energy penalty for the regenerator, with CO2 purity more than 70 percent for a DAC process.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Electrochimica Acta
工程技术-电化学
CiteScore
11.30
自引率
6.10%
发文量
1634
审稿时长
41 days
期刊介绍:
Electrochimica Acta is an international journal. It is intended for the publication of both original work and reviews in the field of electrochemistry. Electrochemistry should be interpreted to mean any of the research fields covered by the Divisions of the International Society of Electrochemistry listed below, as well as emerging scientific domains covered by ISE New Topics Committee.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


