使用二甲基亚砜作为电解质添加剂提高铁硫水溶液电池的容量和稳定性
IF 20.2
1区 材料科学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
探索低成本、高安全性和高容量的金属硫电池是大型存储应用的当务之急。铁(Fe)是一种储量丰富且具有成本效益的元素,作为阳极材料提供了一个很好的选择,它与水电解质中丰富的硫(S)偶联将是一种改变游戏规则的方法。尽管前景看好,但由于铁阳极在水溶液中的副反应和固有的腐蚀倾向,其稳定性限制了其性能。在此,我们探索了二甲亚砜(DMSO)作为高氯酸铁(Fe(ClO4)2)水溶液中Fe- s电池的电解质添加剂,该电池在50 mA g-1时具有1145 mAh g-1的高比容量,并且在2.0和0.5 A g-1电流密度下连续循环400次,分别具有72%和98%的容量保留率,而无需更换Fe阳极。光谱和气相色谱分析结果表明,DMSO的加入抑制了寄生析氢反应(HER)的6.7倍,使铁电极的腐蚀速率降低了2.2倍。分子动力学(MD)模拟表明,DMSO通过氢键与水分子结合,减少了可用于HER和铁电极腐蚀的自由水分子的比例。本文章由计算机程序翻译,如有差异,请以英文原文为准。
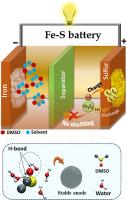

Boosted capacity and stability of aqueous iron-sulfur battery using DMSO as an electrolyte additive
Exploring metal-sulfur batteries with low cost, high safety, and capacity is the need of the hour for large storage applications. Iron (Fe) being a highly abundant and cost-effective element, provides an excellent option as an anode material which on coupling with abundant sulfur (S) in an aqueous electrolyte will be a game-changing approach. Despite a promising outlook, the stability of Fe anode due to side reactions in aqueous electrolytes and inherent corrosion tendencies limit their performance. Herein, we have explored dimethyl sulfoxide (DMSO) as an electrolyte additive in iron percholorate (Fe(ClO4)2 for aqueous Fe-S battery, which exhibited high specific capacity of 1145 mAh g-1 at 50 mA g-1 with remarkable cycling stability for 400 continuous cycles at 2.0 and 0.5 A g-1 current densities with 72 % and 98 % capacity retention respectively without replacing the Fe-anode. The addition of DMSO, suppressed parasitic hydrogen evolution reaction (HER) by 6.7 times and mitigated the corrosion rate of iron electrodes by 2.2 times as evidenced by the spectroscopic and gas chromatography techniques. The molecular dynamics (MD) simulations revealed that DMSO engages the water molecules through hydrogen bonding which reduced the fraction of free water molecules available for HER and corrosion of iron electrodes.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Energy Storage Materials
Materials Science-General Materials Science
CiteScore
33.00
自引率
5.90%
发文量
652
审稿时长
27 days
期刊介绍:
Energy Storage Materials is a global interdisciplinary journal dedicated to sharing scientific and technological advancements in materials and devices for advanced energy storage and related energy conversion, such as in metal-O2 batteries. The journal features comprehensive research articles, including full papers and short communications, as well as authoritative feature articles and reviews by leading experts in the field.
Energy Storage Materials covers a wide range of topics, including the synthesis, fabrication, structure, properties, performance, and technological applications of energy storage materials. Additionally, the journal explores strategies, policies, and developments in the field of energy storage materials and devices for sustainable energy.
Published papers are selected based on their scientific and technological significance, their ability to provide valuable new knowledge, and their relevance to the international research community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


