沉默Sialidase NEU3可通过调节Wnt/β-catenin信号通路抑制EA.hy926细胞的血管生成。
IF 2.2
3区 生物学
Q3 BIOCHEMISTRY & MOLECULAR BIOLOGY
Biochemical and biophysical research communications
Pub Date : 2025-01-01
DOI:10.1016/j.bbrc.2024.151098
引用次数: 0
摘要
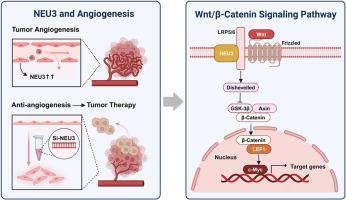
血管生成是肿瘤发展的重要驱动因素,血管内皮细胞的功能受多种因素的影响。肿瘤细胞以唾液酰化异常为特征,其动态平衡依赖于唾液酰转移酶和唾液酰化酶。NEU3是一种质膜相关唾液酸酶,对细胞表面唾液酸化的调节至关重要。我们的研究表明,在EA.hy926细胞中,NEU3是四种唾液酸酶亚型中表达量最高的。沉默NEU3表达导致细胞凋亡和增殖减少,突出了其在调节细胞活性中的重要功能。随后的transwell和试管形成实验表明,NEU3表达的抑制抑制了细胞迁移和血管生成。RNA测序分析进一步阐明,改变EA.hy926细胞NEU3表达可影响Wnt/β-Catenin信号通路和c-Myc水平,从而调节细胞存活和迁移能力,对血管生成起到调节作用。这些发现表明,靶向血管内皮中的NEU3可能是肿瘤抗血管生成治疗的一种有希望的策略。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Sialidase NEU3 silencing inhibits angiogenesis of EA.hy926 cells by regulating Wnt/β-catenin signaling pathway
Angiogenesis significantly drives tumor progression, and the functions of vascular endothelial cells are influenced by various factors. Tumor cells are characterized by abnormal sialylation, and their dynamic balance depends on sialyltransferases and sialidases. NEU3 is a plasma membrane-associated sialidase, vital for the regulation of cell surface sialylation. Our study revealed that, NEU3 is the most abundantly expressed among the four sialidase subtypes in EA.hy926 cells. Silencing NEU3 expression resulted in cell apoptosis and reduced proliferation, highlighting its crucial function in the regulation of cell activity. Subsequent experiments using transwell and tube formation assays demonstrated that the inhibition of NEU3 expression suppressed cell migration and angiogenesis. RNA sequencing analysis further elucidated that altering NEU3 expression in EA.hy926 cells impacts the Wnt/β-Catenin signaling pathway and c-Myc levels, thereby modulating cellular survival and migration capacity and exerting a regulatory effect on angiogenesis. These findings suggest that targeting NEU3 in the vascular endothelium may represent a promising strategy for anti-angiogenic therapy in tumors.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
6.10
自引率
0.00%
发文量
1400
审稿时长
14 days
期刊介绍:
Biochemical and Biophysical Research Communications is the premier international journal devoted to the very rapid dissemination of timely and significant experimental results in diverse fields of biological research. The development of the "Breakthroughs and Views" section brings the minireview format to the journal, and issues often contain collections of special interest manuscripts. BBRC is published weekly (52 issues/year).Research Areas now include: Biochemistry; biophysics; cell biology; developmental biology; immunology
; molecular biology; neurobiology; plant biology and proteomics

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


