对苯二酚增强Cu(II)/过氧单硫酸酯在酸性pH条件下降解磺胺类抗生素
IF 9
1区 工程技术
Q1 ENGINEERING, CHEMICAL
引用次数: 0
摘要
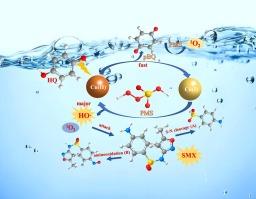
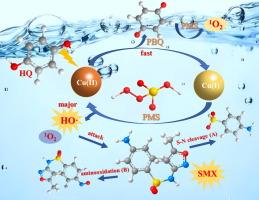
由于过一硫酸铜(PMS)能够产生活性物质,因此人们一直在研究以 Cu(II)/PMS 为媒介活化过一硫酸铜(PMS)来降解污染物的方法。然而,在酸性条件下,由于金属价态的转换速度较慢,Cu(II)/PMS 生成的活性物种产量相对较低,这限制了其应用前景。在此,我们发现在 Cu(II)/PMS 体系中引入对苯二酚(HQ)可通过促进 Cu(II)/Cu(I) 的氧化还原循环来加速 PMS 的活化,从而实现对磺胺类抗生素的高效破坏。在 pH 值为 3.0 的条件下,HQ/Cu(II)/PMS 系统对六种磺胺类抗生素的降解速率常数是 Cu(II)/PMS 系统的 5-8 倍。羟基自由基(HO-)和单线态氧(1O2)都参与了磺胺甲噁唑(SMX,一种具有代表性的磺胺类抗生素)的降解,其中前者的作用最为重要。由于 HQ 中羟基的电子供能能力,HQ 提高了 Cu(II) 对 Cu(I) 的还原,从而促进了 PMS 的分解,在酸性条件下产生更多的 HO-。腐植酸(HA)和低浓度 Cl- 的存在对 SMX 的降解有抑制作用,而高浓度 Cl- 的存在则促进了 SMX 的降解。加入 NO3- 和 SO42- 对 SMX 的去除几乎没有影响。HQ/Cu(II)/PMS 系统对 SMX 的降解途径主要涉及 S-N 裂解和氨基氧化。引入 HQ 后,Cu(II)/PMS 系统对实际废水处理仍有明显的增强效果。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Hydroquinone enhanced Cu(II)/peroxymonosulfate for the degradation of sulfonamide antibiotics under acidic pH conditions
The Cu(II)-mediated activation of peroxymonosulfate (PMS) has been studied for the degradation of contaminants due to its ability to produce reactive species. However, the yield of reactive species generated from Cu(II)/PMS is relatively low under acidic conditions due to the slow conversion rate of metal valence states, which limits its application prospects. Herein, we found that the introduction of hydroquinone (HQ) in the Cu(II)/PMS system can accelerate the activation of PMS by promoting the redox cycling of Cu(II)/Cu(I), thereby achieving efficient destruct of sulfonamide antibiotics. The degradation rate constants of six sulfonamide antibiotics by HQ/Cu(II)/PMS system was 5–8 times of that by Cu(II)/PMS system under pH 3.0. Both hydroxyl radical (HO·) and singlet oxygen (1O2) participated in the degradation of sulfamethoxazole (SMX, a representative sulfonamide antibiotic), and the former played the most important role. HQ improved the reduction of Cu(II) to Cu(I) due to the electron-donating capacity of hydroxyl in HQ, thereby promoting PMS decomposition to produce more HO· under acidic conditions. The presence of humic acid (HA) and low concentration of Cl- presented the inhibitory effect on SMX degradation, while the degradation of SMX was promoted in the presence of high concentration of Cl-. The addition of NO3– and SO42- has almost no effect on the removal of SMX. The degradation pathways of SMX by HQ/Cu(II)/PMS system mainly involved in the S-N cleavage and amino oxidation. The significant enhancement effects were still observed in actual wastewater treatment by Cu(II)/PMS system after introduction of HQ.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Separation and Purification Technology
工程技术-工程:化工
CiteScore
14.00
自引率
12.80%
发文量
2347
审稿时长
43 days
期刊介绍:
Separation and Purification Technology is a premier journal committed to sharing innovative methods for separation and purification in chemical and environmental engineering, encompassing both homogeneous solutions and heterogeneous mixtures. Our scope includes the separation and/or purification of liquids, vapors, and gases, as well as carbon capture and separation techniques. However, it's important to note that methods solely intended for analytical purposes are not within the scope of the journal. Additionally, disciplines such as soil science, polymer science, and metallurgy fall outside the purview of Separation and Purification Technology. Join us in advancing the field of separation and purification methods for sustainable solutions in chemical and environmental engineering.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


