磺胺类抗生素在单原子铜催化剂/过氧单硫酸盐体系中介导电子转移过程的氧化还原电位:磺胺类药物的选择性去除机制
IF 11.3
1区 环境科学与生态学
Q1 ENGINEERING, ENVIRONMENTAL
引用次数: 0
摘要
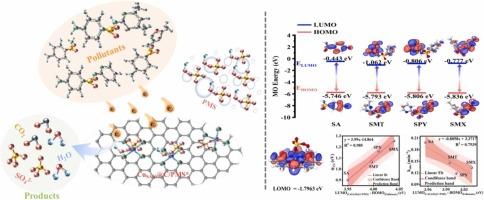
单原子催化剂活化过一硫酸盐(SACs/PMS)体系中目标污染物的氧化行为大多是从负载金属和 SACs 的配位结构方面进行研究的。然而,人们却忽视了污染物的特殊性质所导致的降解行为改变的根源。本文制备了嵌入生物炭(CuSA30@C)中的与四个 N 原子配位的铜原子,以确定磺胺类抗生素在 CuSA30@C/PMS 系统中的选择性降解行为与其自身性质之间的关系。选择了四种具有代表性的磺胺污染物(SAs),并通过测量半波电位(φ1/2)确定了它们的氧化还原电位。结果表明,在 CuSA30@C/PMS 系统中,不同 SA 的 φ1/2 值与其相应的降解速率常数 (kobs) 之间建立了良好的相关性(R2=0.916)。此外,SAs 的 φ1/2 值与 SAs 和 CuSA30@C/PMS 复合物之间的能隙有很好的相关性,进一步证明了 SAs 的氧化还原电位在 CuSA30@C/PMS 体系中的电子转移氧化过程中起着至关重要的作用。这项工作有助于从污染物特性的角度理解磺胺类抗生素在类似芬顿体系中的选择性降解活性,为高效处理磺胺类抗生素废水提供了新思路。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Redox potentials of sulfonamide antibiotics mediating the electron transfer process in single-atom Cu catalyst/peroxymonosulfate system: Selective removal mechanisms for sulfonamides
The oxidative behaviors of target pollutants in single-atom catalysts-activated peroxymonosulfate (SACs/PMS) system has mostly been studied from the loaded metal and coordination structure of SACs. However, the origin of the altered degradation behavior caused by the specific properties of pollutants has been neglected. Herein, Cu atoms coordinated with four N atoms embedded in biochar (CuSA30@C) was prepared to establish the relationship between the selective degradation behavior of sulfonamide antibiotics in CuSA30@C/PMS system and their own properties. Four representative sulfonamide pollutants (SAs) were selected and their redox potentials were determined by measuring half-wave potentials (φ1/2). Results showed that a good correlation (R2=0.916) between the φ1/2 values of different SAs and their corresponding degradation rate constants (kobs) in CuSA30@C/PMS system was established. Additionally, the φ1/2 values of SAs correlate well with the energy gap between SAs and the CuSA30@C/PMS complexes, further proving that the redox potential of SAs played a crucial role for electron-transfer oxidation in CuSA30@C/PMS system. This work contributes to the understanding of the selective degradation activity of sulfonamide antibiotics in Fenton-like systems from the perspective of pollutants properties, and provides new ideas for the efficient treatment of sulfonamide antibiotic wastewater.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Hazardous Materials
工程技术-工程:环境
CiteScore
25.40
自引率
5.90%
发文量
3059
审稿时长
58 days
期刊介绍:
The Journal of Hazardous Materials serves as a global platform for promoting cutting-edge research in the field of Environmental Science and Engineering. Our publication features a wide range of articles, including full-length research papers, review articles, and perspectives, with the aim of enhancing our understanding of the dangers and risks associated with various materials concerning public health and the environment. It is important to note that the term "environmental contaminants" refers specifically to substances that pose hazardous effects through contamination, while excluding those that do not have such impacts on the environment or human health. Moreover, we emphasize the distinction between wastes and hazardous materials in order to provide further clarity on the scope of the journal. We have a keen interest in exploring specific compounds and microbial agents that have adverse effects on the environment.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


