珊瑚状FeCoNi合金/层状双氢氧化物/泡沫镍增强析氧反应的传质
IF 13.2
1区 工程技术
Q1 ENGINEERING, CHEMICAL
引用次数: 0
摘要
许多氧进化反应电催化剂在高电流密度下会失去传质能力,从而导致采用可再生能源制造 "绿色 "氢气成为一个相当大的问题。在此,我们通过在泡沫镍(FCNal/NF)上集成珊瑚状铁钴镍合金/层状双氢氧化物电催化剂,构建了气体疏散配置,可有效避免催化剂在高电流密度下失活。在电化学分水过程中,超亲水/超疏水性表面可减少气泡对电极的粘附。此外,孔隙内自发产生的蛞蝓流可以促进电解质与电化学活性位点之间的接触。因此,经过优化的 FCNal/NF 电极在实验室(1 M KOH,室温)和严格的工业(30 wt% KOH,80 °C)条件下都显示出卓越的催化活性(在 1 M KOH 中 10 mA cm-2 的过电位为 163 mV)和长期的耐久性。在阴离子交换膜电解水(AEMWE)测试中,FCNal/NF(+)||Pt/C/NF(-)电解槽在整体电解水时分别需要 1.73 和 1.86 V 的电压才能达到 1000 和 2000 mA cm-2 的电流密度,这表明它具有工业应用潜力。本文章由计算机程序翻译,如有差异,请以英文原文为准。
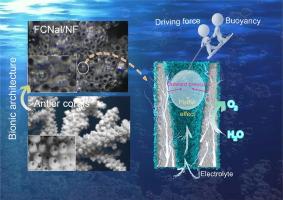
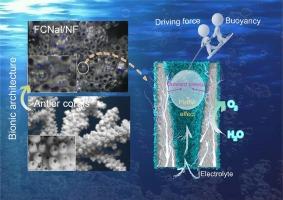
Coral-like FeCoNi alloy/layered double hydroxides/nickel foam for enhancing mass transfer in oxygen evolution reactions
Many oxygen evolution reaction electrocatalysts lose their ability to transfer mass at high current densities, leading to the adoption of renewable power to make “green” hydrogen a considerable problem. Here we construct gas evacuation configurations by integrating coral-like FeCoNi alloy/layered double hydroxides electrocatalysts on nickel foam (FCNal/NF) that can effectively avoid catalyst deactivation in high current density. During electrochemical water splitting, the superhydrophilic/superaerophobic surfaces reduce the adhesion of bubbles to electrodes. Moreover, the spontaneously generated slug flow within pores can facilitate contact between electrolytes and electrochemical active sites. Therefore, the optimized FCNal/NF electrode displays excellent catalytic activity (the overpotential of 166 mV at 10 mA cm−2 in 1 M KOH) with long-term durability under both laboratory (1 M KOH, room temperature) and rigorous industrial (30 wt% KOH, 80 °C) conditions. In the anion exchange membrane water electrolysis (AEMWE) tests, FCNal/NF(+)||Pt/C/NF(−) electrolyzer requires 1.73 and 1.86 V to reach the current density of 1000 and 2000 mA cm−2 for overall water electrolysis, respectively, showing its potential for industrial application.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Chemical Engineering Journal
工程技术-工程:化工
CiteScore
21.70
自引率
9.30%
发文量
6781
审稿时长
2.4 months
期刊介绍:
The Chemical Engineering Journal is an international research journal that invites contributions of original and novel fundamental research. It aims to provide an international platform for presenting original fundamental research, interpretative reviews, and discussions on new developments in chemical engineering. The journal welcomes papers that describe novel theory and its practical application, as well as those that demonstrate the transfer of techniques from other disciplines. It also welcomes reports on carefully conducted experimental work that is soundly interpreted. The main focus of the journal is on original and rigorous research results that have broad significance. The Catalysis section within the Chemical Engineering Journal focuses specifically on Experimental and Theoretical studies in the fields of heterogeneous catalysis, molecular catalysis, and biocatalysis. These studies have industrial impact on various sectors such as chemicals, energy, materials, foods, healthcare, and environmental protection.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


