掺杂镍的石墨烯上 NH 3 和 CH4 的离解机制:调整电子和光学特性
IF 6.3
2区 材料科学
Q2 CHEMISTRY, PHYSICAL
A. Aligayev, U. Jabbarli, U. Samadova, F.J. Dominguez–Gutierrez, S. Papanikolaou, Qing Huang
{"title":"掺杂镍的石墨烯上 NH 3 和 CH4 的离解机制:调整电子和光学特性","authors":"A. Aligayev, U. Jabbarli, U. Samadova, F.J. Dominguez–Gutierrez, S. Papanikolaou, Qing Huang","doi":"10.1016/j.apsusc.2024.162022","DOIUrl":null,"url":null,"abstract":"In this study, we employ a multi-scale computational modeling approach, combining density functional theory (DFT) and self-consistent charge density functional tight binding (SCC-DFTB), to investigate hydrogen (H<span><span style=\"\"></span><span data-mathml='<math xmlns=\"http://www.w3.org/1998/Math/MathML\"><msub is=\"true\"><mrow is=\"true\" /><mrow is=\"true\"><mn is=\"true\">2</mn></mrow></msub></math>' role=\"presentation\" style=\"font-size: 90%; display: inline-block; position: relative;\" tabindex=\"0\"><svg aria-hidden=\"true\" focusable=\"false\" height=\"1.509ex\" role=\"img\" style=\"vertical-align: -0.582ex;\" viewbox=\"0 -399.4 453.9 649.8\" width=\"1.054ex\" xmlns:xlink=\"http://www.w3.org/1999/xlink\"><g fill=\"currentColor\" stroke=\"currentColor\" stroke-width=\"0\" transform=\"matrix(1 0 0 -1 0 0)\"><g is=\"true\"><g is=\"true\"></g><g is=\"true\" transform=\"translate(0,-150)\"><g is=\"true\"><use transform=\"scale(0.707)\" xlink:href=\"#MJMAIN-32\"></use></g></g></g></g></svg><span role=\"presentation\"><math xmlns=\"http://www.w3.org/1998/Math/MathML\"><msub is=\"true\"><mrow is=\"true\"></mrow><mrow is=\"true\"><mn is=\"true\">2</mn></mrow></msub></math></span></span><script type=\"math/mml\"><math><msub is=\"true\"><mrow is=\"true\"></mrow><mrow is=\"true\"><mn is=\"true\">2</mn></mrow></msub></math></script></span>) production and dissociation mechanisms from ammonia (NH<span><span style=\"\"></span><span data-mathml='<math xmlns=\"http://www.w3.org/1998/Math/MathML\"><msub is=\"true\"><mrow is=\"true\" /><mrow is=\"true\"><mn is=\"true\">3</mn></mrow></msub></math>' role=\"presentation\" style=\"font-size: 90%; display: inline-block; position: relative;\" tabindex=\"0\"><svg aria-hidden=\"true\" focusable=\"false\" height=\"1.509ex\" role=\"img\" style=\"vertical-align: -0.582ex;\" viewbox=\"0 -399.4 453.9 649.8\" width=\"1.054ex\" xmlns:xlink=\"http://www.w3.org/1999/xlink\"><g fill=\"currentColor\" stroke=\"currentColor\" stroke-width=\"0\" transform=\"matrix(1 0 0 -1 0 0)\"><g is=\"true\"><g is=\"true\"></g><g is=\"true\" transform=\"translate(0,-150)\"><g is=\"true\"><use transform=\"scale(0.707)\" xlink:href=\"#MJMAIN-33\"></use></g></g></g></g></svg><span role=\"presentation\"><math xmlns=\"http://www.w3.org/1998/Math/MathML\"><msub is=\"true\"><mrow is=\"true\"></mrow><mrow is=\"true\"><mn is=\"true\">3</mn></mrow></msub></math></span></span><script type=\"math/mml\"><math><msub is=\"true\"><mrow is=\"true\"></mrow><mrow is=\"true\"><mn is=\"true\">3</mn></mrow></msub></math></script></span>) and methane (CH<span><span style=\"\"></span><span data-mathml='<math xmlns=\"http://www.w3.org/1998/Math/MathML\"><msub is=\"true\"><mrow is=\"true\" /><mrow is=\"true\"><mn is=\"true\">4</mn></mrow></msub></math>' role=\"presentation\" style=\"font-size: 90%; display: inline-block; position: relative;\" tabindex=\"0\"><svg aria-hidden=\"true\" focusable=\"false\" height=\"1.509ex\" role=\"img\" style=\"vertical-align: -0.582ex;\" viewbox=\"0 -399.4 453.9 649.8\" width=\"1.054ex\" xmlns:xlink=\"http://www.w3.org/1999/xlink\"><g fill=\"currentColor\" stroke=\"currentColor\" stroke-width=\"0\" transform=\"matrix(1 0 0 -1 0 0)\"><g is=\"true\"><g is=\"true\"></g><g is=\"true\" transform=\"translate(0,-150)\"><g is=\"true\"><use transform=\"scale(0.707)\" xlink:href=\"#MJMAIN-34\"></use></g></g></g></g></svg><span role=\"presentation\"><math xmlns=\"http://www.w3.org/1998/Math/MathML\"><msub is=\"true\"><mrow is=\"true\"></mrow><mrow is=\"true\"><mn is=\"true\">4</mn></mrow></msub></math></span></span><script type=\"math/mml\"><math><msub is=\"true\"><mrow is=\"true\"></mrow><mrow is=\"true\"><mn is=\"true\">4</mn></mrow></msub></math></script></span>) on pristine and nickel-doped graphene. These two-dimensional materials hold significant potential for applications in advanced gas sensing and catalysis. Our analysis reveals that Ni-doped graphene, validated through work function calculations, is a promising material for gas separation and hydrogen production. The samples with adsorbed molecules are characterized by calculating chemical potetential, chemical hardness, electronegativyt, electrophilicity, vibrational frequencies, adsorbtion and Gibbs energies by DFT calculations. Methane molecules preferentially adsorb at the hexagonal ring centers of graphene, while ammonia interacts more strongly with carbon atoms, highlighting distinct molecular doping mechanisms for CH<span><span style=\"\"></span><span data-mathml='<math xmlns=\"http://www.w3.org/1998/Math/MathML\"><msub is=\"true\"><mrow is=\"true\" /><mrow is=\"true\"><mn is=\"true\">4</mn></mrow></msub></math>' role=\"presentation\" style=\"font-size: 90%; display: inline-block; position: relative;\" tabindex=\"0\"><svg aria-hidden=\"true\" focusable=\"false\" height=\"1.509ex\" role=\"img\" style=\"vertical-align: -0.582ex;\" viewbox=\"0 -399.4 453.9 649.8\" width=\"1.054ex\" xmlns:xlink=\"http://www.w3.org/1999/xlink\"><g fill=\"currentColor\" stroke=\"currentColor\" stroke-width=\"0\" transform=\"matrix(1 0 0 -1 0 0)\"><g is=\"true\"><g is=\"true\"></g><g is=\"true\" transform=\"translate(0,-150)\"><g is=\"true\"><use transform=\"scale(0.707)\" xlink:href=\"#MJMAIN-34\"></use></g></g></g></g></svg><span role=\"presentation\"><math xmlns=\"http://www.w3.org/1998/Math/MathML\"><msub is=\"true\"><mrow is=\"true\"></mrow><mrow is=\"true\"><mn is=\"true\">4</mn></mrow></msub></math></span></span><script type=\"math/mml\"><math><msub is=\"true\"><mrow is=\"true\"></mrow><mrow is=\"true\"><mn is=\"true\">4</mn></mrow></msub></math></script></span> and NH<span><span style=\"\"></span><span data-mathml='<math xmlns=\"http://www.w3.org/1998/Math/MathML\"><msub is=\"true\"><mrow is=\"true\" /><mrow is=\"true\"><mn is=\"true\">3</mn></mrow></msub></math>' role=\"presentation\" style=\"font-size: 90%; display: inline-block; position: relative;\" tabindex=\"0\"><svg aria-hidden=\"true\" focusable=\"false\" height=\"1.509ex\" role=\"img\" style=\"vertical-align: -0.582ex;\" viewbox=\"0 -399.4 453.9 649.8\" width=\"1.054ex\" xmlns:xlink=\"http://www.w3.org/1999/xlink\"><g fill=\"currentColor\" stroke=\"currentColor\" stroke-width=\"0\" transform=\"matrix(1 0 0 -1 0 0)\"><g is=\"true\"><g is=\"true\"></g><g is=\"true\" transform=\"translate(0,-150)\"><g is=\"true\"><use transform=\"scale(0.707)\" xlink:href=\"#MJMAIN-33\"></use></g></g></g></g></svg><span role=\"presentation\"><math xmlns=\"http://www.w3.org/1998/Math/MathML\"><msub is=\"true\"><mrow is=\"true\"></mrow><mrow is=\"true\"><mn is=\"true\">3</mn></mrow></msub></math></span></span><script type=\"math/mml\"><math><msub is=\"true\"><mrow is=\"true\"></mrow><mrow is=\"true\"><mn is=\"true\">3</mn></mrow></msub></math></script></span>. Dynamic simulations show that CH<span><span style=\"\"></span><span data-mathml='<math xmlns=\"http://www.w3.org/1998/Math/MathML\"><msub is=\"true\"><mrow is=\"true\" /><mrow is=\"true\"><mn is=\"true\">4</mn></mrow></msub></math>' role=\"presentation\" style=\"font-size: 90%; display: inline-block; position: relative;\" tabindex=\"0\"><svg aria-hidden=\"true\" focusable=\"false\" height=\"1.509ex\" role=\"img\" style=\"vertical-align: -0.582ex;\" viewbox=\"0 -399.4 453.9 649.8\" width=\"1.054ex\" xmlns:xlink=\"http://www.w3.org/1999/xlink\"><g fill=\"currentColor\" stroke=\"currentColor\" stroke-width=\"0\" transform=\"matrix(1 0 0 -1 0 0)\"><g is=\"true\"><g is=\"true\"></g><g is=\"true\" transform=\"translate(0,-150)\"><g is=\"true\"><use transform=\"scale(0.707)\" xlink:href=\"#MJMAIN-34\"></use></g></g></g></g></svg><span role=\"presentation\"><math xmlns=\"http://www.w3.org/1998/Math/MathML\"><msub is=\"true\"><mrow is=\"true\"></mrow><mrow is=\"true\"><mn is=\"true\">4</mn></mrow></msub></math></span></span><script type=\"math/mml\"><math><msub is=\"true\"><mrow is=\"true\"></mrow><mrow is=\"true\"><mn is=\"true\">4</mn></mrow></msub></math></script></span> splits into CH<span><span style=\"\"></span><span data-mathml='<math xmlns=\"http://www.w3.org/1998/Math/MathML\"><msub is=\"true\"><mrow is=\"true\" /><mrow is=\"true\"><mn is=\"true\">3</mn></mrow></msub></math>' role=\"presentation\" style=\"font-size: 90%; display: inline-block; position: relative;\" tabindex=\"0\"><svg aria-hidden=\"true\" focusable=\"false\" height=\"1.509ex\" role=\"img\" style=\"vertical-align: -0.582ex;\" viewbox=\"0 -399.4 453.9 649.8\" width=\"1.054ex\" xmlns:xlink=\"http://www.w3.org/1999/xlink\"><g fill=\"currentColor\" stroke=\"currentColor\" stroke-width=\"0\" transform=\"matrix(1 0 0 -1 0 0)\"><g is=\"true\"><g is=\"true\"></g><g is=\"true\" transform=\"translate(0,-150)\"><g is=\"true\"><use transform=\"scale(0.707)\" xlink:href=\"#MJMAIN-33\"></use></g></g></g></g></svg><span role=\"presentation\"><math xmlns=\"http://www.w3.org/1998/Math/MathML\"><msub is=\"true\"><mrow is=\"true\"></mrow><mrow is=\"true\"><mn is=\"true\">3</mn></mrow></msub></math></span></span><script type=\"math/mml\"><math><msub is=\"true\"><mrow is=\"true\"></mrow><mrow is=\"true\"><mn is=\"true\">3</mn></mrow></msub></math></script></span>+H, with Ni-doped graphene facilitating enhanced hydrogen transmission, while NH<span><span style=\"\"></span><span data-mathml='<math xmlns=\"http://www.w3.org/1998/Math/MathML\"><msub is=\"true\"><mrow is=\"true\" /><mrow is=\"true\"><mn is=\"true\">3</mn></mrow></msub></math>' role=\"presentation\" style=\"font-size: 90%; display: inline-block; position: relative;\" tabindex=\"0\"><svg aria-hidden=\"true\" focusable=\"false\" height=\"1.509ex\" role=\"img\" style=\"vertical-align: -0.582ex;\" viewbox=\"0 -399.4 453.9 649.8\" width=\"1.054ex\" xmlns:xlink=\"http://www.w3.org/1999/xlink\"><g fill=\"currentColor\" stroke=\"currentColor\" stroke-width=\"0\" transform=\"matrix(1 0 0 -1 0 0)\"><g is=\"true\"><g is=\"true\"></g><g is=\"true\" transform=\"translate(0,-150)\"><g is=\"true\"><use transform=\"scale(0.707)\" xlink:href=\"#MJMAIN-33\"></use></g></g></g></g></svg><span role=\"presentation\"><math xmlns=\"http://www.w3.org/1998/Math/MathML\"><msub is=\"true\"><mrow is=\"true\"></mrow><mrow is=\"true\"><mn is=\"true\">3</mn></mrow></msub></math></span></span><script type=\"math/mml\"><math><msub is=\"true\"><mrow is=\"true\"></mrow><mrow is=\"true\"><mn is=\"true\">3</mn></mrow></msub></math></script></span> dissociates into NH<span><span style=\"\"></span><span data-mathml='<math xmlns=\"http://www.w3.org/1998/Math/MathML\"><msub is=\"true\"><mrow is=\"true\" /><mrow is=\"true\"><mn is=\"true\">2</mn></mrow></msub></math>' role=\"presentation\" style=\"font-size: 90%; display: inline-block; position: relative;\" tabindex=\"0\"><svg aria-hidden=\"true\" focusable=\"false\" height=\"1.509ex\" role=\"img\" style=\"vertical-align: -0.582ex;\" viewbox=\"0 -399.4 453.9 649.8\" width=\"1.054ex\" xmlns:xlink=\"http://www.w3.org/1999/xlink\"><g fill=\"currentColor\" stroke=\"currentColor\" stroke-width=\"0\" transform=\"matrix(1 0 0 -1 0 0)\"><g is=\"true\"><g is=\"true\"></g><g is=\"true\" transform=\"translate(0,-150)\"><g is=\"true\"><use transform=\"scale(0.707)\" xlink:href=\"#MJMAIN-32\"></use></g></g></g></g></svg><span role=\"presentation\"><math xmlns=\"http://www.w3.org/1998/Math/MathML\"><msub is=\"true\"><mrow is=\"true\"></mrow><mrow is=\"true\"><mn is=\"true\">2</mn></mrow></msub></math></span></span><script type=\"math/mml\"><math><msub is=\"true\"><mrow is=\"true\"></mrow><mrow is=\"true\"><mn is=\"true\">2</mn></mrow></msub></math></script></span>+H, which may lead to N<span><span style=\"\"></span><span data-mathml='<math xmlns=\"http://www.w3.org/1998/Math/MathML\"><msub is=\"true\"><mrow is=\"true\" /><mrow is=\"true\"><mn is=\"true\">2</mn></mrow></msub></math>' role=\"presentation\" style=\"font-size: 90%; display: inline-block; position: relative;\" tabindex=\"0\"><svg aria-hidden=\"true\" focusable=\"false\" height=\"1.509ex\" role=\"img\" style=\"vertical-align: -0.582ex;\" viewbox=\"0 -399.4 453.9 649.8\" width=\"1.054ex\" xmlns:xlink=\"http://www.w3.org/1999/xlink\"><g fill=\"currentColor\" stroke=\"currentColor\" stroke-width=\"0\" transform=\"matrix(1 0 0 -1 0 0)\"><g is=\"true\"><g is=\"true\"></g><g is=\"true\" transform=\"translate(0,-150)\"><g is=\"true\"><use transform=\"scale(0.707)\" xlink:href=\"#MJMAIN-32\"></use></g></g></g></g></svg><span role=\"presentation\"><math xmlns=\"http://www.w3.org/1998/Math/MathML\"><msub is=\"true\"><mrow is=\"true\"></mrow><mrow is=\"true\"><mn is=\"true\">2</mn></mrow></msub></math></span></span><script type=\"math/mml\"><math><msub is=\"true\"><mrow is=\"true\"></mrow><mrow is=\"true\"><mn is=\"true\">2</mn></mrow></msub></math></script></span>H<span><span style=\"\"></span><span data-mathml='<math xmlns=\"http://www.w3.org/1998/Math/MathML\"><msub is=\"true\"><mrow is=\"true\" /><mrow is=\"true\"><mn is=\"true\">4</mn></mrow></msub></math>' role=\"presentation\" style=\"font-size: 90%; display: inline-block; position: relative;\" tabindex=\"0\"><svg aria-hidden=\"true\" focusable=\"false\" height=\"1.509ex\" role=\"img\" style=\"vertical-align: -0.582ex;\" viewbox=\"0 -399.4 453.9 649.8\" width=\"1.054ex\" xmlns:xlink=\"http://www.w3.org/1999/xlink\"><g fill=\"currentColor\" stroke=\"currentColor\" stroke-width=\"0\" transform=\"matrix(1 0 0 -1 0 0)\"><g is=\"true\"><g is=\"true\"></g><g is=\"true\" transform=\"translate(0,-150)\"><g is=\"true\"><use transform=\"scale(0.707)\" xlink:href=\"#MJMAIN-34\"></use></g></g></g></g></svg><span role=\"presentation\"><math xmlns=\"http://www.w3.org/1998/Math/MathML\"><msub is=\"true\"><mrow is=\"true\"></mrow><mrow is=\"true\"><mn is=\"true\">4</mn></mrow></msub></math></span></span><script type=\"math/mml\"><math><msub is=\"true\"><mrow is=\"true\"></mrow><mrow is=\"true\"><mn is=\"true\">4</mn></mrow></msub></math></script></span> formation. Our non-equilibrium Green’s function (NEGF) simulations demonstrate increased H-atom transmission on Ni-doped graphene during gas interactions. These findings suggest that Ni-doped graphene is superior to pristine graphene for applications in gas separation, hydrogen production, and high-sensitivity sensors.","PeriodicalId":247,"journal":{"name":"Applied Surface Science","volume":"8 1","pages":""},"PeriodicalIF":6.3000,"publicationDate":"2024-12-14","publicationTypes":"Journal Article","fieldsOfStudy":null,"isOpenAccess":false,"openAccessPdf":"","citationCount":"0","resultStr":"{\"title\":\"Dissociative mechanism from NH 3 and CH4 on Ni-doped graphene: Tuning electronic and optical properties\",\"authors\":\"A. Aligayev, U. Jabbarli, U. Samadova, F.J. Dominguez–Gutierrez, S. Papanikolaou, Qing Huang\",\"doi\":\"10.1016/j.apsusc.2024.162022\",\"DOIUrl\":null,\"url\":null,\"abstract\":\"In this study, we employ a multi-scale computational modeling approach, combining density functional theory (DFT) and self-consistent charge density functional tight binding (SCC-DFTB), to investigate hydrogen (H<span><span style=\\\"\\\"></span><span data-mathml='<math xmlns=\\\"http://www.w3.org/1998/Math/MathML\\\"><msub is=\\\"true\\\"><mrow is=\\\"true\\\" /><mrow is=\\\"true\\\"><mn is=\\\"true\\\">2</mn></mrow></msub></math>' role=\\\"presentation\\\" style=\\\"font-size: 90%; display: inline-block; position: relative;\\\" tabindex=\\\"0\\\"><svg aria-hidden=\\\"true\\\" focusable=\\\"false\\\" height=\\\"1.509ex\\\" role=\\\"img\\\" style=\\\"vertical-align: -0.582ex;\\\" viewbox=\\\"0 -399.4 453.9 649.8\\\" width=\\\"1.054ex\\\" xmlns:xlink=\\\"http://www.w3.org/1999/xlink\\\"><g fill=\\\"currentColor\\\" stroke=\\\"currentColor\\\" stroke-width=\\\"0\\\" transform=\\\"matrix(1 0 0 -1 0 0)\\\"><g is=\\\"true\\\"><g is=\\\"true\\\"></g><g is=\\\"true\\\" transform=\\\"translate(0,-150)\\\"><g is=\\\"true\\\"><use transform=\\\"scale(0.707)\\\" xlink:href=\\\"#MJMAIN-32\\\"></use></g></g></g></g></svg><span role=\\\"presentation\\\"><math xmlns=\\\"http://www.w3.org/1998/Math/MathML\\\"><msub is=\\\"true\\\"><mrow is=\\\"true\\\"></mrow><mrow is=\\\"true\\\"><mn is=\\\"true\\\">2</mn></mrow></msub></math></span></span><script type=\\\"math/mml\\\"><math><msub is=\\\"true\\\"><mrow is=\\\"true\\\"></mrow><mrow is=\\\"true\\\"><mn is=\\\"true\\\">2</mn></mrow></msub></math></script></span>) production and dissociation mechanisms from ammonia (NH<span><span style=\\\"\\\"></span><span data-mathml='<math xmlns=\\\"http://www.w3.org/1998/Math/MathML\\\"><msub is=\\\"true\\\"><mrow is=\\\"true\\\" /><mrow is=\\\"true\\\"><mn is=\\\"true\\\">3</mn></mrow></msub></math>' role=\\\"presentation\\\" style=\\\"font-size: 90%; display: inline-block; position: relative;\\\" tabindex=\\\"0\\\"><svg aria-hidden=\\\"true\\\" focusable=\\\"false\\\" height=\\\"1.509ex\\\" role=\\\"img\\\" style=\\\"vertical-align: -0.582ex;\\\" viewbox=\\\"0 -399.4 453.9 649.8\\\" width=\\\"1.054ex\\\" xmlns:xlink=\\\"http://www.w3.org/1999/xlink\\\"><g fill=\\\"currentColor\\\" stroke=\\\"currentColor\\\" stroke-width=\\\"0\\\" transform=\\\"matrix(1 0 0 -1 0 0)\\\"><g is=\\\"true\\\"><g is=\\\"true\\\"></g><g is=\\\"true\\\" transform=\\\"translate(0,-150)\\\"><g is=\\\"true\\\"><use transform=\\\"scale(0.707)\\\" xlink:href=\\\"#MJMAIN-33\\\"></use></g></g></g></g></svg><span role=\\\"presentation\\\"><math xmlns=\\\"http://www.w3.org/1998/Math/MathML\\\"><msub is=\\\"true\\\"><mrow is=\\\"true\\\"></mrow><mrow is=\\\"true\\\"><mn is=\\\"true\\\">3</mn></mrow></msub></math></span></span><script type=\\\"math/mml\\\"><math><msub is=\\\"true\\\"><mrow is=\\\"true\\\"></mrow><mrow is=\\\"true\\\"><mn is=\\\"true\\\">3</mn></mrow></msub></math></script></span>) and methane (CH<span><span style=\\\"\\\"></span><span data-mathml='<math xmlns=\\\"http://www.w3.org/1998/Math/MathML\\\"><msub is=\\\"true\\\"><mrow is=\\\"true\\\" /><mrow is=\\\"true\\\"><mn is=\\\"true\\\">4</mn></mrow></msub></math>' role=\\\"presentation\\\" style=\\\"font-size: 90%; display: inline-block; position: relative;\\\" tabindex=\\\"0\\\"><svg aria-hidden=\\\"true\\\" focusable=\\\"false\\\" height=\\\"1.509ex\\\" role=\\\"img\\\" style=\\\"vertical-align: -0.582ex;\\\" viewbox=\\\"0 -399.4 453.9 649.8\\\" width=\\\"1.054ex\\\" xmlns:xlink=\\\"http://www.w3.org/1999/xlink\\\"><g fill=\\\"currentColor\\\" stroke=\\\"currentColor\\\" stroke-width=\\\"0\\\" transform=\\\"matrix(1 0 0 -1 0 0)\\\"><g is=\\\"true\\\"><g is=\\\"true\\\"></g><g is=\\\"true\\\" transform=\\\"translate(0,-150)\\\"><g is=\\\"true\\\"><use transform=\\\"scale(0.707)\\\" xlink:href=\\\"#MJMAIN-34\\\"></use></g></g></g></g></svg><span role=\\\"presentation\\\"><math xmlns=\\\"http://www.w3.org/1998/Math/MathML\\\"><msub is=\\\"true\\\"><mrow is=\\\"true\\\"></mrow><mrow is=\\\"true\\\"><mn is=\\\"true\\\">4</mn></mrow></msub></math></span></span><script type=\\\"math/mml\\\"><math><msub is=\\\"true\\\"><mrow is=\\\"true\\\"></mrow><mrow is=\\\"true\\\"><mn is=\\\"true\\\">4</mn></mrow></msub></math></script></span>) on pristine and nickel-doped graphene. These two-dimensional materials hold significant potential for applications in advanced gas sensing and catalysis. Our analysis reveals that Ni-doped graphene, validated through work function calculations, is a promising material for gas separation and hydrogen production. The samples with adsorbed molecules are characterized by calculating chemical potetential, chemical hardness, electronegativyt, electrophilicity, vibrational frequencies, adsorbtion and Gibbs energies by DFT calculations. Methane molecules preferentially adsorb at the hexagonal ring centers of graphene, while ammonia interacts more strongly with carbon atoms, highlighting distinct molecular doping mechanisms for CH<span><span style=\\\"\\\"></span><span data-mathml='<math xmlns=\\\"http://www.w3.org/1998/Math/MathML\\\"><msub is=\\\"true\\\"><mrow is=\\\"true\\\" /><mrow is=\\\"true\\\"><mn is=\\\"true\\\">4</mn></mrow></msub></math>' role=\\\"presentation\\\" style=\\\"font-size: 90%; display: inline-block; position: relative;\\\" tabindex=\\\"0\\\"><svg aria-hidden=\\\"true\\\" focusable=\\\"false\\\" height=\\\"1.509ex\\\" role=\\\"img\\\" style=\\\"vertical-align: -0.582ex;\\\" viewbox=\\\"0 -399.4 453.9 649.8\\\" width=\\\"1.054ex\\\" xmlns:xlink=\\\"http://www.w3.org/1999/xlink\\\"><g fill=\\\"currentColor\\\" stroke=\\\"currentColor\\\" stroke-width=\\\"0\\\" transform=\\\"matrix(1 0 0 -1 0 0)\\\"><g is=\\\"true\\\"><g is=\\\"true\\\"></g><g is=\\\"true\\\" transform=\\\"translate(0,-150)\\\"><g is=\\\"true\\\"><use transform=\\\"scale(0.707)\\\" xlink:href=\\\"#MJMAIN-34\\\"></use></g></g></g></g></svg><span role=\\\"presentation\\\"><math xmlns=\\\"http://www.w3.org/1998/Math/MathML\\\"><msub is=\\\"true\\\"><mrow is=\\\"true\\\"></mrow><mrow is=\\\"true\\\"><mn is=\\\"true\\\">4</mn></mrow></msub></math></span></span><script type=\\\"math/mml\\\"><math><msub is=\\\"true\\\"><mrow is=\\\"true\\\"></mrow><mrow is=\\\"true\\\"><mn is=\\\"true\\\">4</mn></mrow></msub></math></script></span> and NH<span><span style=\\\"\\\"></span><span data-mathml='<math xmlns=\\\"http://www.w3.org/1998/Math/MathML\\\"><msub is=\\\"true\\\"><mrow is=\\\"true\\\" /><mrow is=\\\"true\\\"><mn is=\\\"true\\\">3</mn></mrow></msub></math>' role=\\\"presentation\\\" style=\\\"font-size: 90%; display: inline-block; position: relative;\\\" tabindex=\\\"0\\\"><svg aria-hidden=\\\"true\\\" focusable=\\\"false\\\" height=\\\"1.509ex\\\" role=\\\"img\\\" style=\\\"vertical-align: -0.582ex;\\\" viewbox=\\\"0 -399.4 453.9 649.8\\\" width=\\\"1.054ex\\\" xmlns:xlink=\\\"http://www.w3.org/1999/xlink\\\"><g fill=\\\"currentColor\\\" stroke=\\\"currentColor\\\" stroke-width=\\\"0\\\" transform=\\\"matrix(1 0 0 -1 0 0)\\\"><g is=\\\"true\\\"><g is=\\\"true\\\"></g><g is=\\\"true\\\" transform=\\\"translate(0,-150)\\\"><g is=\\\"true\\\"><use transform=\\\"scale(0.707)\\\" xlink:href=\\\"#MJMAIN-33\\\"></use></g></g></g></g></svg><span role=\\\"presentation\\\"><math xmlns=\\\"http://www.w3.org/1998/Math/MathML\\\"><msub is=\\\"true\\\"><mrow is=\\\"true\\\"></mrow><mrow is=\\\"true\\\"><mn is=\\\"true\\\">3</mn></mrow></msub></math></span></span><script type=\\\"math/mml\\\"><math><msub is=\\\"true\\\"><mrow is=\\\"true\\\"></mrow><mrow is=\\\"true\\\"><mn is=\\\"true\\\">3</mn></mrow></msub></math></script></span>. Dynamic simulations show that CH<span><span style=\\\"\\\"></span><span data-mathml='<math xmlns=\\\"http://www.w3.org/1998/Math/MathML\\\"><msub is=\\\"true\\\"><mrow is=\\\"true\\\" /><mrow is=\\\"true\\\"><mn is=\\\"true\\\">4</mn></mrow></msub></math>' role=\\\"presentation\\\" style=\\\"font-size: 90%; display: inline-block; position: relative;\\\" tabindex=\\\"0\\\"><svg aria-hidden=\\\"true\\\" focusable=\\\"false\\\" height=\\\"1.509ex\\\" role=\\\"img\\\" style=\\\"vertical-align: -0.582ex;\\\" viewbox=\\\"0 -399.4 453.9 649.8\\\" width=\\\"1.054ex\\\" xmlns:xlink=\\\"http://www.w3.org/1999/xlink\\\"><g fill=\\\"currentColor\\\" stroke=\\\"currentColor\\\" stroke-width=\\\"0\\\" transform=\\\"matrix(1 0 0 -1 0 0)\\\"><g is=\\\"true\\\"><g is=\\\"true\\\"></g><g is=\\\"true\\\" transform=\\\"translate(0,-150)\\\"><g is=\\\"true\\\"><use transform=\\\"scale(0.707)\\\" xlink:href=\\\"#MJMAIN-34\\\"></use></g></g></g></g></svg><span role=\\\"presentation\\\"><math xmlns=\\\"http://www.w3.org/1998/Math/MathML\\\"><msub is=\\\"true\\\"><mrow is=\\\"true\\\"></mrow><mrow is=\\\"true\\\"><mn is=\\\"true\\\">4</mn></mrow></msub></math></span></span><script type=\\\"math/mml\\\"><math><msub is=\\\"true\\\"><mrow is=\\\"true\\\"></mrow><mrow is=\\\"true\\\"><mn is=\\\"true\\\">4</mn></mrow></msub></math></script></span> splits into CH<span><span style=\\\"\\\"></span><span data-mathml='<math xmlns=\\\"http://www.w3.org/1998/Math/MathML\\\"><msub is=\\\"true\\\"><mrow is=\\\"true\\\" /><mrow is=\\\"true\\\"><mn is=\\\"true\\\">3</mn></mrow></msub></math>' role=\\\"presentation\\\" style=\\\"font-size: 90%; display: inline-block; position: relative;\\\" tabindex=\\\"0\\\"><svg aria-hidden=\\\"true\\\" focusable=\\\"false\\\" height=\\\"1.509ex\\\" role=\\\"img\\\" style=\\\"vertical-align: -0.582ex;\\\" viewbox=\\\"0 -399.4 453.9 649.8\\\" width=\\\"1.054ex\\\" xmlns:xlink=\\\"http://www.w3.org/1999/xlink\\\"><g fill=\\\"currentColor\\\" stroke=\\\"currentColor\\\" stroke-width=\\\"0\\\" transform=\\\"matrix(1 0 0 -1 0 0)\\\"><g is=\\\"true\\\"><g is=\\\"true\\\"></g><g is=\\\"true\\\" transform=\\\"translate(0,-150)\\\"><g is=\\\"true\\\"><use transform=\\\"scale(0.707)\\\" xlink:href=\\\"#MJMAIN-33\\\"></use></g></g></g></g></svg><span role=\\\"presentation\\\"><math xmlns=\\\"http://www.w3.org/1998/Math/MathML\\\"><msub is=\\\"true\\\"><mrow is=\\\"true\\\"></mrow><mrow is=\\\"true\\\"><mn is=\\\"true\\\">3</mn></mrow></msub></math></span></span><script type=\\\"math/mml\\\"><math><msub is=\\\"true\\\"><mrow is=\\\"true\\\"></mrow><mrow is=\\\"true\\\"><mn is=\\\"true\\\">3</mn></mrow></msub></math></script></span>+H, with Ni-doped graphene facilitating enhanced hydrogen transmission, while NH<span><span style=\\\"\\\"></span><span data-mathml='<math xmlns=\\\"http://www.w3.org/1998/Math/MathML\\\"><msub is=\\\"true\\\"><mrow is=\\\"true\\\" /><mrow is=\\\"true\\\"><mn is=\\\"true\\\">3</mn></mrow></msub></math>' role=\\\"presentation\\\" style=\\\"font-size: 90%; display: inline-block; position: relative;\\\" tabindex=\\\"0\\\"><svg aria-hidden=\\\"true\\\" focusable=\\\"false\\\" height=\\\"1.509ex\\\" role=\\\"img\\\" style=\\\"vertical-align: -0.582ex;\\\" viewbox=\\\"0 -399.4 453.9 649.8\\\" width=\\\"1.054ex\\\" xmlns:xlink=\\\"http://www.w3.org/1999/xlink\\\"><g fill=\\\"currentColor\\\" stroke=\\\"currentColor\\\" stroke-width=\\\"0\\\" transform=\\\"matrix(1 0 0 -1 0 0)\\\"><g is=\\\"true\\\"><g is=\\\"true\\\"></g><g is=\\\"true\\\" transform=\\\"translate(0,-150)\\\"><g is=\\\"true\\\"><use transform=\\\"scale(0.707)\\\" xlink:href=\\\"#MJMAIN-33\\\"></use></g></g></g></g></svg><span role=\\\"presentation\\\"><math xmlns=\\\"http://www.w3.org/1998/Math/MathML\\\"><msub is=\\\"true\\\"><mrow is=\\\"true\\\"></mrow><mrow is=\\\"true\\\"><mn is=\\\"true\\\">3</mn></mrow></msub></math></span></span><script type=\\\"math/mml\\\"><math><msub is=\\\"true\\\"><mrow is=\\\"true\\\"></mrow><mrow is=\\\"true\\\"><mn is=\\\"true\\\">3</mn></mrow></msub></math></script></span> dissociates into NH<span><span style=\\\"\\\"></span><span data-mathml='<math xmlns=\\\"http://www.w3.org/1998/Math/MathML\\\"><msub is=\\\"true\\\"><mrow is=\\\"true\\\" /><mrow is=\\\"true\\\"><mn is=\\\"true\\\">2</mn></mrow></msub></math>' role=\\\"presentation\\\" style=\\\"font-size: 90%; display: inline-block; position: relative;\\\" tabindex=\\\"0\\\"><svg aria-hidden=\\\"true\\\" focusable=\\\"false\\\" height=\\\"1.509ex\\\" role=\\\"img\\\" style=\\\"vertical-align: -0.582ex;\\\" viewbox=\\\"0 -399.4 453.9 649.8\\\" width=\\\"1.054ex\\\" xmlns:xlink=\\\"http://www.w3.org/1999/xlink\\\"><g fill=\\\"currentColor\\\" stroke=\\\"currentColor\\\" stroke-width=\\\"0\\\" transform=\\\"matrix(1 0 0 -1 0 0)\\\"><g is=\\\"true\\\"><g is=\\\"true\\\"></g><g is=\\\"true\\\" transform=\\\"translate(0,-150)\\\"><g is=\\\"true\\\"><use transform=\\\"scale(0.707)\\\" xlink:href=\\\"#MJMAIN-32\\\"></use></g></g></g></g></svg><span role=\\\"presentation\\\"><math xmlns=\\\"http://www.w3.org/1998/Math/MathML\\\"><msub is=\\\"true\\\"><mrow is=\\\"true\\\"></mrow><mrow is=\\\"true\\\"><mn is=\\\"true\\\">2</mn></mrow></msub></math></span></span><script type=\\\"math/mml\\\"><math><msub is=\\\"true\\\"><mrow is=\\\"true\\\"></mrow><mrow is=\\\"true\\\"><mn is=\\\"true\\\">2</mn></mrow></msub></math></script></span>+H, which may lead to N<span><span style=\\\"\\\"></span><span data-mathml='<math xmlns=\\\"http://www.w3.org/1998/Math/MathML\\\"><msub is=\\\"true\\\"><mrow is=\\\"true\\\" /><mrow is=\\\"true\\\"><mn is=\\\"true\\\">2</mn></mrow></msub></math>' role=\\\"presentation\\\" style=\\\"font-size: 90%; display: inline-block; position: relative;\\\" tabindex=\\\"0\\\"><svg aria-hidden=\\\"true\\\" focusable=\\\"false\\\" height=\\\"1.509ex\\\" role=\\\"img\\\" style=\\\"vertical-align: -0.582ex;\\\" viewbox=\\\"0 -399.4 453.9 649.8\\\" width=\\\"1.054ex\\\" xmlns:xlink=\\\"http://www.w3.org/1999/xlink\\\"><g fill=\\\"currentColor\\\" stroke=\\\"currentColor\\\" stroke-width=\\\"0\\\" transform=\\\"matrix(1 0 0 -1 0 0)\\\"><g is=\\\"true\\\"><g is=\\\"true\\\"></g><g is=\\\"true\\\" transform=\\\"translate(0,-150)\\\"><g is=\\\"true\\\"><use transform=\\\"scale(0.707)\\\" xlink:href=\\\"#MJMAIN-32\\\"></use></g></g></g></g></svg><span role=\\\"presentation\\\"><math xmlns=\\\"http://www.w3.org/1998/Math/MathML\\\"><msub is=\\\"true\\\"><mrow is=\\\"true\\\"></mrow><mrow is=\\\"true\\\"><mn is=\\\"true\\\">2</mn></mrow></msub></math></span></span><script type=\\\"math/mml\\\"><math><msub is=\\\"true\\\"><mrow is=\\\"true\\\"></mrow><mrow is=\\\"true\\\"><mn is=\\\"true\\\">2</mn></mrow></msub></math></script></span>H<span><span style=\\\"\\\"></span><span data-mathml='<math xmlns=\\\"http://www.w3.org/1998/Math/MathML\\\"><msub is=\\\"true\\\"><mrow is=\\\"true\\\" /><mrow is=\\\"true\\\"><mn is=\\\"true\\\">4</mn></mrow></msub></math>' role=\\\"presentation\\\" style=\\\"font-size: 90%; display: inline-block; position: relative;\\\" tabindex=\\\"0\\\"><svg aria-hidden=\\\"true\\\" focusable=\\\"false\\\" height=\\\"1.509ex\\\" role=\\\"img\\\" style=\\\"vertical-align: -0.582ex;\\\" viewbox=\\\"0 -399.4 453.9 649.8\\\" width=\\\"1.054ex\\\" xmlns:xlink=\\\"http://www.w3.org/1999/xlink\\\"><g fill=\\\"currentColor\\\" stroke=\\\"currentColor\\\" stroke-width=\\\"0\\\" transform=\\\"matrix(1 0 0 -1 0 0)\\\"><g is=\\\"true\\\"><g is=\\\"true\\\"></g><g is=\\\"true\\\" transform=\\\"translate(0,-150)\\\"><g is=\\\"true\\\"><use transform=\\\"scale(0.707)\\\" xlink:href=\\\"#MJMAIN-34\\\"></use></g></g></g></g></svg><span role=\\\"presentation\\\"><math xmlns=\\\"http://www.w3.org/1998/Math/MathML\\\"><msub is=\\\"true\\\"><mrow is=\\\"true\\\"></mrow><mrow is=\\\"true\\\"><mn is=\\\"true\\\">4</mn></mrow></msub></math></span></span><script type=\\\"math/mml\\\"><math><msub is=\\\"true\\\"><mrow is=\\\"true\\\"></mrow><mrow is=\\\"true\\\"><mn is=\\\"true\\\">4</mn></mrow></msub></math></script></span> formation. Our non-equilibrium Green’s function (NEGF) simulations demonstrate increased H-atom transmission on Ni-doped graphene during gas interactions. These findings suggest that Ni-doped graphene is superior to pristine graphene for applications in gas separation, hydrogen production, and high-sensitivity sensors.\",\"PeriodicalId\":247,\"journal\":{\"name\":\"Applied Surface Science\",\"volume\":\"8 1\",\"pages\":\"\"},\"PeriodicalIF\":6.3000,\"publicationDate\":\"2024-12-14\",\"publicationTypes\":\"Journal Article\",\"fieldsOfStudy\":null,\"isOpenAccess\":false,\"openAccessPdf\":\"\",\"citationCount\":\"0\",\"resultStr\":null,\"platform\":\"Semanticscholar\",\"paperid\":null,\"PeriodicalName\":\"Applied Surface Science\",\"FirstCategoryId\":\"88\",\"ListUrlMain\":\"https://doi.org/10.1016/j.apsusc.2024.162022\",\"RegionNum\":2,\"RegionCategory\":\"材料科学\",\"ArticlePicture\":[],\"TitleCN\":null,\"AbstractTextCN\":null,\"PMCID\":null,\"EPubDate\":\"\",\"PubModel\":\"\",\"JCR\":\"Q2\",\"JCRName\":\"CHEMISTRY, PHYSICAL\",\"Score\":null,\"Total\":0}","platform":"Semanticscholar","paperid":null,"PeriodicalName":"Applied Surface Science","FirstCategoryId":"88","ListUrlMain":"https://doi.org/10.1016/j.apsusc.2024.162022","RegionNum":2,"RegionCategory":"材料科学","ArticlePicture":[],"TitleCN":null,"AbstractTextCN":null,"PMCID":null,"EPubDate":"","PubModel":"","JCR":"Q2","JCRName":"CHEMISTRY, PHYSICAL","Score":null,"Total":0}
引用次数: 0
摘要
在本研究中,我们采用多尺度计算建模方法,结合密度泛函理论(DFT)和自洽电荷密度泛函紧密结合(SCC-DFTB),研究了原始石墨烯和掺镍石墨烯上氨气(NH33)和甲烷(CH44)的氢气(H22)产生和解离机制。这些二维材料在先进气体传感和催化方面具有巨大的应用潜力。我们的分析表明,通过功函数计算验证,掺镍石墨烯是一种很有前途的气体分离和制氢材料。通过 DFT 计算化学势、化学硬度、电负性、亲电性、振动频率、吸附能和吉布斯能,对吸附了分子的样品进行了表征。甲烷分子优先吸附在石墨烯的六角环中心,而氨则与碳原子发生更强烈的相互作用,凸显了 CH44 和 NH33 不同的分子掺杂机制。动态模拟显示,CH44 会分裂成 CH33+H,掺杂镍的石墨烯会促进氢的传输,而 NH33 则会解离成 NH22+H,这可能会导致 N22H44 的形成。我们的非平衡格林函数(NEGF)模拟表明,在气体相互作用过程中,掺镍石墨烯上的氢原子传输能力增强。这些发现表明,掺镍石墨烯在气体分离、制氢和高灵敏度传感器方面的应用优于原始石墨烯。本文章由计算机程序翻译,如有差异,请以英文原文为准。
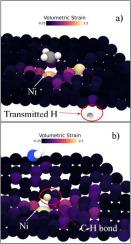
Dissociative mechanism from NH 3 and CH4 on Ni-doped graphene: Tuning electronic and optical properties
In this study, we employ a multi-scale computational modeling approach, combining density functional theory (DFT) and self-consistent charge density functional tight binding (SCC-DFTB), to investigate hydrogen (H) production and dissociation mechanisms from ammonia (NH) and methane (CH) on pristine and nickel-doped graphene. These two-dimensional materials hold significant potential for applications in advanced gas sensing and catalysis. Our analysis reveals that Ni-doped graphene, validated through work function calculations, is a promising material for gas separation and hydrogen production. The samples with adsorbed molecules are characterized by calculating chemical potetential, chemical hardness, electronegativyt, electrophilicity, vibrational frequencies, adsorbtion and Gibbs energies by DFT calculations. Methane molecules preferentially adsorb at the hexagonal ring centers of graphene, while ammonia interacts more strongly with carbon atoms, highlighting distinct molecular doping mechanisms for CH and NH. Dynamic simulations show that CH splits into CH+H, with Ni-doped graphene facilitating enhanced hydrogen transmission, while NH dissociates into NH+H, which may lead to NH formation. Our non-equilibrium Green’s function (NEGF) simulations demonstrate increased H-atom transmission on Ni-doped graphene during gas interactions. These findings suggest that Ni-doped graphene is superior to pristine graphene for applications in gas separation, hydrogen production, and high-sensitivity sensors.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Applied Surface Science
工程技术-材料科学:膜
CiteScore
12.50
自引率
7.50%
发文量
3393
审稿时长
67 days
期刊介绍:
Applied Surface Science covers topics contributing to a better understanding of surfaces, interfaces, nanostructures and their applications. The journal is concerned with scientific research on the atomic and molecular level of material properties determined with specific surface analytical techniques and/or computational methods, as well as the processing of such structures.
×
引用
GB/T 7714-2015
复制
MLA
复制
APA
复制
导出至
BibTeX
EndNote
RefMan
NoteFirst
NoteExpress
请完成安全验证×
微信好友
朋友圈
QQ好友
复制链接
取消

已复制链接
快去分享给好友吧!
我知道了

点击右上角分享
相关文献
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:
