氨在富镍氢氧化物前驱体微反应器快速结晶动力学中的作用
IF 3.9
3区 工程技术
Q2 ENGINEERING, CHEMICAL
引用次数: 0
摘要
前驱体的形态和微观结构是制备高质量NCM阴极的关键,因为它们具有遗传关系。但前驱体中存在大量的硫酸盐,不利于富镍正极材料的电化学性能。本文提出了在微反应器中加入0.24 M氨水合成Ni0.90Co0.05Mn0.05(OH)2前驱体的有效硫酸盐还原方法。Ni(OH)2-氨体系中硫酸盐含量较低是由于硫酸盐吸附的形成能较高(ENi(OH)2-am- so42 - = 7.483 eV vs ENi(OH)2- so42 - = 3.451 eV)。结果表明:氨能促进初生颗粒(81.5 nm)的生长,使前驱体中杂质硫酸盐的含量从0.80%降至0.28%;此外,制备的阴极材料在1C下具有190.4 mAh g-1的高比容量和优异的Li+扩散系数。该研究揭示了氨在快速共沉淀中的作用,为富镍阴极的快速合成工艺提供了指导。本文章由计算机程序翻译,如有差异,请以英文原文为准。
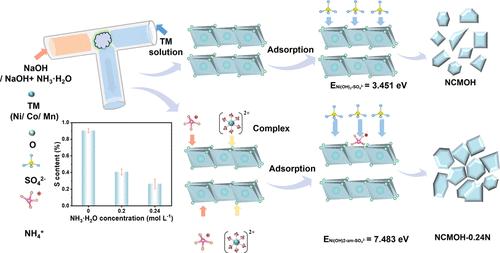
Revealing the Role of Ammonia in the Rapid Crystallization Kinetics for Ni-Rich Hydroxide Precursors via Microreactors
The morphology and microstructure of precursors are the key to the preparation of high-quality NCM cathodes due to their inheritance relationship. However, a large amount of sulfate exists in the precursors, to the detriment of the electrochemical performance of the Ni-rich cathode materials. Herein, an effective method for sulfate reduction was proposed for the synthesis of Ni0.90Co0.05Mn0.05(OH)2 precursor by adding 0.24 M ammonia in the microreactor. The less sulfate in the Ni(OH)2 with ammonia system is attributed to the higher formation energy of sulfate adsorption (ENi(OH)2-am-SO42– = 7.483 eV vs ENi(OH)2-SO42– = 3.451 eV). The results demonstrated that ammonia can promote the growth of primary particles (81.5 nm) and reduce the content of impurity sulfate from 0.80 to 0.28% in the precursors. Moreover, the prepared cathode materials exhibit a high specific capacity of 190.4 mAh g–1 at 1C and superior Li+ diffusion coefficients. This study sheds light on the role of ammonia in rapid coprecipitation and provides guidance for the rapid synthesis process of Ni-rich cathodes.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Industrial & Engineering Chemistry Research
工程技术-工程:化工
CiteScore
7.40
自引率
7.10%
发文量
1467
审稿时长
2.8 months
期刊介绍:
ndustrial & Engineering Chemistry, with variations in title and format, has been published since 1909 by the American Chemical Society. Industrial & Engineering Chemistry Research is a weekly publication that reports industrial and academic research in the broad fields of applied chemistry and chemical engineering with special focus on fundamentals, processes, and products.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


