设计等温自然对流为主的电化学电池:实验验证
IF 3.9
3区 工程技术
Q2 ENGINEERING, CHEMICAL
引用次数: 0
摘要
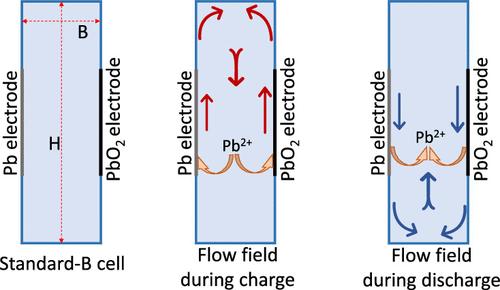
电化学电池(如可溶性铅氧化还原电池)中的沉积/溶解氧化还原反应会在电解质中产生强烈的自然对流。在这些系统中,流场是由浓度梯度驱动的密度梯度形成的,由于施密特数(与普朗特数相比)的数值天然较大,因此在热系统中遇到的雷利数范围并不常见。在这项工作中,我们首次报告了可溶性氧化还原铅化学电池自然对流电解质溶液的直接 PIV 测量结果,并将其与现有模型的预测结果进行了比较。我们通过对不同电池宽度的低纵横比电池进行实验,直接测试了二维流动假设。将模型预测的单个电极电位与在这项工作中进行的测量结果进行比较,结果表明拟合参数是适当的,在引入贯流电池后保持不变。通过比较几种新配置的预测值和实验测量值,我们进一步测试了该模型。这些新配置包括高电池、电极间隙较大的电池、电极间活性区顶部或下方有额外空间的电池,以及充放电间歇性混合。这些新配置进一步揭示了基于可溶性铅氧化还原化学的电池中多种物理因素是如何相互作用的。我们对电池电位曲线的测量验证了所有情况下的模型预测。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Designing Isothermal Natural Convection Dominated Electrochemical Cells: Experimental Validation
Deposition/dissolution redox reactions in electrochemical cells, such as soluble lead redox batteries, induce strong natural convection flow in the electrolyte. The Rayleigh number range encountered in these systems where the flow field is set up due to concentration-gradient-driven density gradients is uncommon in thermal systems, by virtue of naturally large values of Schmidt number, as compared to Prandtl number. In this work, we report the first direct PIV measurements of natural convection electrolyte solutions for soluble lead redox chemistry cells and compare them with the predictions of the existing models. We directly test the assumption of 2-dimensional flow by experimenting with low aspect ratio cells of different cell widths. A comparison of the model-predicted individual electrode potentials with measurements carried out in this work shows the adequacy of the fitted parameters, which have remained unchanged after their introduction in the context of a flow-through battery. We further tested the model by comparing predictions for several new configurations with experimental measurements. The new configurations include tall cells, cells with significant electrode gaps, cells provided with extra space at the top or below the active zone between electrodes, and intermittent mixing between charge and discharge. The new configurations bring further insights into how the multiple physics interact in soluble lead redox chemistry-based cells. Our measurements of cell potential profiles validate the model predictions in all cases.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Industrial & Engineering Chemistry Research
工程技术-工程:化工
CiteScore
7.40
自引率
7.10%
发文量
1467
审稿时长
2.8 months
期刊介绍:
ndustrial & Engineering Chemistry, with variations in title and format, has been published since 1909 by the American Chemical Society. Industrial & Engineering Chemistry Research is a weekly publication that reports industrial and academic research in the broad fields of applied chemistry and chemical engineering with special focus on fundamentals, processes, and products.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


