TMSN3引发叔酰胺的电化学单脱烷基反应
IF 3.6
2区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
摘要
酰胺的n -脱烷基是生物体和有机合成化学中的一个普遍过程,但目前尚未找到有效的化学方法。在此,我们报告了一种电化学方法用于广泛的叔酰胺的单脱烷基反应,包括苯酰胺、烷基酰胺、内酰胺和磺胺。该反应以TMSN3为引发剂,在温和条件下顺利进行,不局限于去乙基化或去甲基化。该协议实现了大规模合成,为合成有机化学提供了有价值的工具。本文章由计算机程序翻译,如有差异,请以英文原文为准。
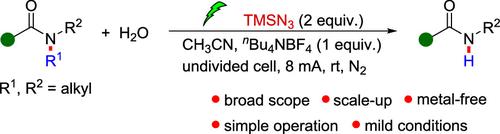
TMSN3 Initiated Electrochemical Mono-Dealkylation of Tertiary Amides
N-Dealkylation of amides is a general process in living organisms and organic synthetic chemistry, but an efficient chemical approach for this transformation has not been explored. Herein, we report an electrochemical method for the monodealkylation of a wide range of tertiary amides, including benzamides, alkyl amides, lactams, and sulfonamides. The reaction proceeds smoothly under mild conditions using TMSN3 as the initiator and is not limited to deethylation or demethylation. This protocol enables the large synthesis, providing a valuable tool for synthetic organic chemistry.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Organic Chemistry
化学-有机化学
CiteScore
6.20
自引率
11.10%
发文量
1467
审稿时长
2 months
期刊介绍:
Journal of Organic Chemistry welcomes original contributions of fundamental research in all branches of the theory and practice of organic chemistry. In selecting manuscripts for publication, the editors place emphasis on the quality and novelty of the work, as well as the breadth of interest to the organic chemistry community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


