通过TrxR1和Gpx4抑制双重调节凋亡和铁致凋亡通路,解读太阳酰胺治疗乳腺癌的综合构效关系
IF 6.8
1区 医学
Q1 CHEMISTRY, MEDICINAL
引用次数: 0
摘要
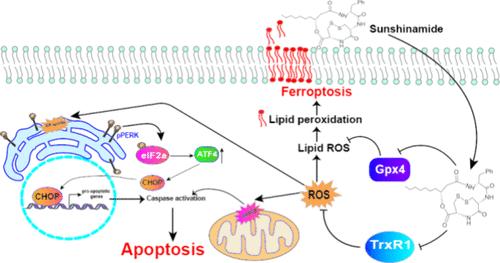
环表肽类化合物太阳神酰胺在抑制癌细胞增殖方面具有巨大潜力。我们之前的研究确定了太阳神酰胺的全合成和抗癌特性。然而,深入了解太阳神酰胺的结构-活性关系(SAR)仍是当务之急。在这项研究中,我们旨在阐明太阳神酰胺在体外和体内的 SAR 和作用机理。SAR 研究证实了双环和二硫化物在太阳神酰胺抗癌活性中的关键作用。我们最近的研究发现,太阳神酰胺靶向 TrxR1,导致产生 ROS 和 ER 应激介导的细胞凋亡,同时还通过靶向 Gpx4 促进脂质过氧化,使癌细胞易受铁变态反应的影响。在体内,实验证明了太阳神酰胺通过诱导细胞凋亡和铁凋亡来降低肿瘤生长的有效性。太阳神酰胺在诱导细胞凋亡和铁蛋白沉降方面的双重功效使其成为乳腺癌治疗的一个有希望的候选药物,从而应对化疗耐药性的挑战。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Deciphering the Comprehensive Structure–Activity Relationship of Sunshinamide for Breast Cancer Therapy through Dual Modulation of Apoptotic and Ferroptotic Pathways via TrxR1 and Gpx4 Inhibition
Sunshinamide, a cyclodepsipeptide, has demonstrated significant potential in inhibiting cancer cell proliferation. Our prior research established the total synthesis and anticancer properties of sunshinamide. However, a deeper understanding of the structure–activity relationship (SAR) of sunshinamide remained imperative. In this study, we aimed to elucidate the SAR and mechanistic insights underlying sunshinamide action, both in vitro and in vivo. SAR studies confirm the crucial roles of both the bicyclic-ring and disulfide moiety in the anticancer activity of sunshinamide. Our recent findings unveil that sunshinamide targets TrxR1, leading to ROS generation and ER-stress-mediated apoptosis, while also promoting lipid peroxidation by targeting Gpx4, rendering cancer cells vulnerable to ferroptosis. In vivo, experiments demonstrated the effectiveness of sunshinamide in reducing tumor growth by inducing both apoptosis and ferroptosis. The dual efficacy of sunshinamide in eliciting apoptosis and ferroptosis positions it as a promising candidate for breast cancer therapy, addressing the challenge of chemoresistance.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Medicinal Chemistry
医学-医药化学
CiteScore
4.00
自引率
11.00%
发文量
804
审稿时长
1.9 months
期刊介绍:
The Journal of Medicinal Chemistry is a prestigious biweekly peer-reviewed publication that focuses on the multifaceted field of medicinal chemistry. Since its inception in 1959 as the Journal of Medicinal and Pharmaceutical Chemistry, it has evolved to become a cornerstone in the dissemination of research findings related to the design, synthesis, and development of therapeutic agents.
The Journal of Medicinal Chemistry is recognized for its significant impact in the scientific community, as evidenced by its 2022 impact factor of 7.3. This metric reflects the journal's influence and the importance of its content in shaping the future of drug discovery and development. The journal serves as a vital resource for chemists, pharmacologists, and other researchers interested in the molecular mechanisms of drug action and the optimization of therapeutic compounds.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


