一种高亲和选择性半乳糖凝集素-3小鼠工具化合物在小鼠癌症模型中的开发和表征
IF 6.8
1区 医学
Q1 CHEMISTRY, MEDICINAL
引用次数: 0
摘要
在过去的几年里,随着几种新型化合物的开发,人们对半乳糖凝集素-3作为癌症和纤维化领域的药物靶点的兴趣越来越大。首个口服半乳糖凝集素-3抑制剂GB1211 (h-半乳糖凝集素-3 Kd = 0.025 μM)目前处于2期临床试验阶段。由于人和小鼠半乳糖凝集素-3的结构差异,大多数高效的人半乳糖凝集素-3抑制剂(m-半乳糖凝集素-3 Kd = 0.77 μM)对小鼠半乳糖凝集素-3的亲和力显著降低。在给药GB1211至100 mg/kg的小鼠中进行的药代动力学实验结果显示,游离血浆水平低于m-半乳糖凝集素- 3kd,这与在人类中观察到的数据不可比较。为了更好地支持转化为临床研究,开发了一种新的改进的小鼠半凝集素-3工具化合物GB2095。在体内同基因小鼠癌症模型中使用这种新化合物可减少乳腺癌和黑色素瘤的生长。本文章由计算机程序翻译,如有差异,请以英文原文为准。
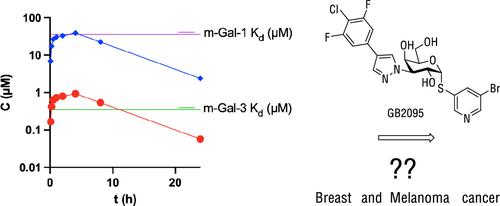
Development and Characterization of a High-Affinity Selective Galectin-3 Mouse Tool Compound in Mouse Models of Cancer
The interest in galectin-3 as a drug target in the cancer and fibrosis space has grown during the past few years with several new classes of compounds being developed. The first orally available galectin-3 inhibitor, GB1211 (h-galectin-3 Kd = 0.025 μM), is currently in phase 2 clinical trials. Due to structural differences between human and mouse galectin-3 a significant reduction in mouse galectin-3 affinity is observed for most highly potent human galectin-3 inhibitors including GB1211 (m-galectin-3 Kd = 0.77 μM). Pharmacokinetic experiments in mouse dosing GB1211 up to 100 mg/kg results in free plasma levels below m-galectin-3 Kd, which is not comparable to the data observed in humans. To better support translation into clinical studies, a new improved mouse galectin-3 tool compound, GB2095, was developed. Dosing this new compound in in vivo syngeneic mouse models of cancer resulted in reduction of the growth of breast and melanoma cancers.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Medicinal Chemistry
医学-医药化学
CiteScore
4.00
自引率
11.00%
发文量
804
审稿时长
1.9 months
期刊介绍:
The Journal of Medicinal Chemistry is a prestigious biweekly peer-reviewed publication that focuses on the multifaceted field of medicinal chemistry. Since its inception in 1959 as the Journal of Medicinal and Pharmaceutical Chemistry, it has evolved to become a cornerstone in the dissemination of research findings related to the design, synthesis, and development of therapeutic agents.
The Journal of Medicinal Chemistry is recognized for its significant impact in the scientific community, as evidenced by its 2022 impact factor of 7.3. This metric reflects the journal's influence and the importance of its content in shaping the future of drug discovery and development. The journal serves as a vital resource for chemists, pharmacologists, and other researchers interested in the molecular mechanisms of drug action and the optimization of therapeutic compounds.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


