代谢纳米调节剂诱导铁下垂并改变代谢物流动以逆转免疫抑制肿瘤微环境
IF 16
1区 材料科学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
肿瘤细胞异常的能量和物质代谢途径通过劫持非恶性细胞的资源,从而建立起有利于肿瘤进展的代谢物流,为肿瘤细胞的增殖提供了重要支持。肿瘤细胞的这种代谢适应性会进一步调节免疫环境,最终形成以耐药性和免疫抑制为特征的肿瘤微环境。协同调节能量和物质代谢途径可能是一种很好的抗肿瘤治疗模式。然而,由于代谢趋同,在不损害其他细胞功能的情况下,选择性地调节肿瘤细胞的异常代谢至关重要。小分子药物能够靶向多种生物大分子进行抗肿瘤治疗,但其应用受到不良毒性的限制。构建纳米药物递送系统可以改善其特性,并允许加入多种药物,从而发挥协同抗肿瘤作用。在这项研究中,我们利用药物共轭多枝聚合物开发了一种双药联合给药系统,通过利用合成致死途径来调节肿瘤细胞代谢,从而实现安全有效的抗肿瘤治疗。通过纳米颗粒同时递送阿达帕林和厄拉斯汀,可以调节肿瘤细胞的物质和能量代谢。这种纳米粒子结构可实现肿瘤组织靶向和响应性药物释放,改变肿瘤细胞内的代谢物流,有效杀死肿瘤细胞。此外,纳米粒子还能逆转肿瘤免疫抑制微环境,实现从单细胞调控到全病灶调控。本文章由计算机程序翻译,如有差异,请以英文原文为准。
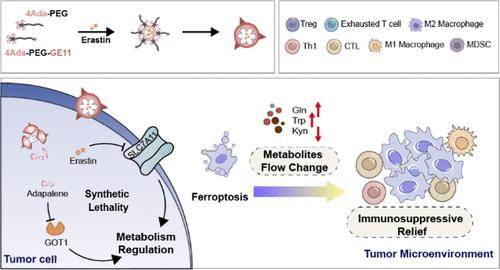
Metabolic Nanoregulators Induce Ferroptosis and Change Metabolite Flow to Reverse Immunosuppressive Tumor Microenvironment
Aberrant energy and substance metabolic pathways of tumor cells critically support tumor cell proliferation by hijacking the resources from nonmalignant cells, thereby establishing a metabolite flow favorable to tumor progression. This metabolic adaptation of tumor cells further modulates the immune landscape, ultimately creating a tumor microenvironment characterized by drug resistance and immunosuppression. The synergistic regulation of energy and substance metabolic pathways might be a good antitumor therapeutic paradigm. However, due to the metabolic convergence, it is crucial to selectively modulate the aberrant metabolism of tumor cells without compromising the functionality of other cells. Small-molecule drugs have the ability to target a wide range of biomolecules for antitumor therapy, but their application is limited by undesirable toxicities. Constructing nanodrug delivery systems can improve their properties and allow for the inclusion of multiple drugs, thereby exerting synergistic antitumor effects. In this study, we developed a two-drug codelivery system using drugs-conjugated multibranched polymers to modulate tumor cell metabolism by exploiting synthetic lethal pathways for safe and effective antitumor therapy. By delivery of adapalene and erastin simultaneously through nanoparticles, the material and energy metabolism of tumor cells can be regulated. This nanoparticle construction achieves tumor tissue targeting and responsive drug release, alters metabolite flow within tumor cells, and effectively kills tumor cells. Additionally, the nanoparticles can reverse the tumor immunosuppressive microenvironment, starting from single-cell regulation to whole-lesion control.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

ACS Nano
工程技术-材料科学:综合
CiteScore
26.00
自引率
4.10%
发文量
1627
审稿时长
1.7 months
期刊介绍:
ACS Nano, published monthly, serves as an international forum for comprehensive articles on nanoscience and nanotechnology research at the intersections of chemistry, biology, materials science, physics, and engineering. The journal fosters communication among scientists in these communities, facilitating collaboration, new research opportunities, and advancements through discoveries. ACS Nano covers synthesis, assembly, characterization, theory, and simulation of nanostructures, nanobiotechnology, nanofabrication, methods and tools for nanoscience and nanotechnology, and self- and directed-assembly. Alongside original research articles, it offers thorough reviews, perspectives on cutting-edge research, and discussions envisioning the future of nanoscience and nanotechnology.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


