PdZn表面和负载PdZn簇上CO2加氢生成HCOOH的比较DFT研究
IF 6.9
2区 材料科学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
要设计出具有更高活性、稳定性和选择性的催化剂,对基于支撑簇的异相催化剂进行改性非常重要。将 Pd 与 Zn 合金并以 ZrO2 为支撑是设计 CO2 加氢制 HCOOH 催化剂的一种很有前景的方法,但活性催化位点的性质和机理仍然未知。我们研究了两种具有代表性的模型:亚纳米团簇 Pd5Zn/ZrO2 和 PdZn(101) 表面。DFT 计算与微动力学模拟相结合,确定了反应的最佳结构和配置。与 PdZn(101)表面相比,Pd5Zn/ZrO2 的吸附和中间产物的形成更为稳定。此外,从热力学和动力学角度来看,甲酸酯路线更有可能在 PdZn(101) 表面上进行。相比之下,被支持的 Pd5Zn/ZrO2 团簇更倾向于羧基途径,团簇与支持物之间的界面位点被认为是更稳定的构型。电子结构分析揭示了中间体形成过程中过渡态的性质,特别是 Pd5Zn/ZrO2 上 Pd 和 Zn 边缘原子对羧基途径选择性的作用。最后,微动力学模拟结果的比较显示,在中高温条件下,Pd5Zn/ZrO2 比 PdZn(101) 表面更倾向于形成 HCOOH。本文章由计算机程序翻译,如有差异,请以英文原文为准。
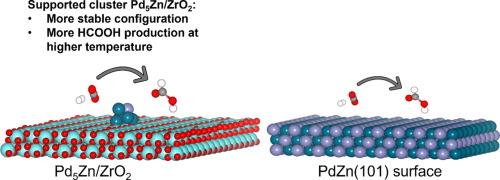
CO2 hydrogenation to HCOOH on PdZn surface and supported PdZn Cluster: A Comparative DFT study
Modifying heterogeneous catalysts for supported cluster-based types is important to design catalysts with better activity, stability, and selectivity. Alloying Pd with Zn and supported by ZrO2 is a promising way to design catalysts for CO2 hydrogenation to HCOOH, but the nature of the active catalytic sites and the mechanism remain unknown. Two representative models have been investigated: subnanometer cluster Pd5Zn/ZrO2 and PdZn(101) surface. DFT calculations combined with microkinetic simulations are used to identify the optimum structure and configurations for the reaction. Compared to the PdZn(101) surface, the Pd5Zn/ZrO2 offers much more stable adsorption and formation of intermediate species. Moreover, the formate route is more likely to proceed on PdZn(101) surface from the viewpoint of thermodynamic and kinetic. In contrast, the supported Pd5Zn/ZrO2 cluster prefers the carboxyl pathway, where the interface site between cluster-support is ascribed to a far more stable configuration. Electronic structure analysis reveals the nature of the transition state on intermediate formation, particularly the role of Pd and Zn edge atoms on the selectivity towards the carboxyl pathway on Pd5Zn/ZrO2. Finally, the comparison of microkinetic simulation results shows a preference for HCOOH formation on Pd5Zn/ZrO2 than PdZn(101) surface at medium to higher temperature.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Applied Surface Science
工程技术-材料科学:膜
CiteScore
12.50
自引率
7.50%
发文量
3393
审稿时长
67 days
期刊介绍:
Applied Surface Science covers topics contributing to a better understanding of surfaces, interfaces, nanostructures and their applications. The journal is concerned with scientific research on the atomic and molecular level of material properties determined with specific surface analytical techniques and/or computational methods, as well as the processing of such structures.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


