钌络合物催化芳香二胺脱氨缩聚反应
IF 4.1
2区 化学
Q2 POLYMER SCIENCE
引用次数: 0
摘要
本研究探索了一种开创性的催化反应,以获得功能性聚合物和有价值的副产物。以RuCl2(PPh3)3为催化剂,与1,4-丁炔二醇结合,激活芳香二胺中的C-N和N-H键。这种活化引发脱氨化缩聚,产生具有吡咯基的芳香多胺和氨作为副产物。缩聚过程中产生的氨可以在冷水中捕获。用奈斯勒试剂法用紫外-可见光谱法证实了缩聚过程中氨的生成。随后,芳香族多胺通过与NH基团的1,4-丁烷磺酸和异氰酸丁酯的聚合物反应进一步功能化。该方法得到的产物分别含有n -丁基磺酸钠和n -丁酰胺基团。前者表现出单离子电导率。利用密度泛函理论计算和2H核磁共振波谱研究了AD中钌催化的N-H和C-N键活化以及末端吡啶基形成的潜在反应机制。本文章由计算机程序翻译,如有差异,请以英文原文为准。
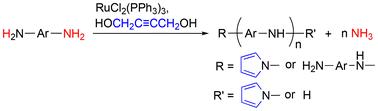

Ruthenium-complex-catalysed de-ammonification polycondensation of aromatic diamines†
This study explores a pioneering catalytic reaction to obtain functional polymers and valuable byproducts. Using RuCl2(PPh3)3 as a catalyst activates the C–N and N–H bonds in aromatic diamines, when combined with 1,4-butynediol. This activation initiates de-ammonification polycondensation, resulting in aromatic polyamines with a pyrrolyl end group and ammonia as a byproduct. The ammonia generated during the polycondensation process can be captured in cold water. The generation of ammonia during polycondensation was confirmed by UV-vis spectroscopy using the Nessler's reagent method. Subsequently, the aromatic polyamines were further functionalised via polymer reactions with 1,4-butanesultone and butyl isocyanate in the NH group. This yielded products with pendant sodium N-butylsulfonate and N-butylamide groups, respectively. The former exhibited a single-ion conductivity. Potential reaction mechanisms involving Ru-catalysed N–H and C–N bond activation in AD, along with the formation of terminal pyrrolyl groups, were investigated using density functional theorycalculations and 2H NMR spectroscopy.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Polymer Chemistry
POLYMER SCIENCE-
CiteScore
8.60
自引率
8.70%
发文量
535
审稿时长
1.7 months
期刊介绍:
Polymer Chemistry welcomes submissions in all areas of polymer science that have a strong focus on macromolecular chemistry. Manuscripts may cover a broad range of fields, yet no direct application focus is required.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


