结合激子-等离子体相互作用和可编程DNA循环扩增用于5 -羟色胺的电化学发光/光电化学传感。
IF 6.1
1区 化学
Q1 CHEMISTRY, ANALYTICAL
引用次数: 0
摘要
研制了一种新型电化学发光(ECL)/光电化学(PEC)双模生物传感器,用于血清素的灵敏检测。在该系统中,PEC信号由CdS量子点(QDs)产生,而ECL信号来自L-Au NPs(鲁米诺修饰的Au纳米颗粒),从而避免了通常由两种信号使用相同材料引起的外部干扰和信号波动。目标5 -羟色胺的存在引发了磁珠(MB)上的非酶催化链位移反应(TSDR),随后在感应界面上发生了催化发夹组装(CHA),导致许多L-Au NPs聚集。强激子-等离子体相互作用(EPI)诱导CdS量子点和Au NPs之间的能量转移,引起光电流的显著抑制。此外,这种设计保证了ECL和PEC以相反的方式响应,并且没有检测到背景ECL信号,从而大大提高了生物传感器的灵敏度。该传感器具有5 ~ 1 μM的宽线性范围,检测限为1.6 pM,也可用于血清和尿液样品的检测,结果令人满意。该传感策略具有高灵敏度、选择性、准确性和信号稳定性等优点,有助于疾病诊断和神经递质基础研究。本文章由计算机程序翻译,如有差异,请以英文原文为准。
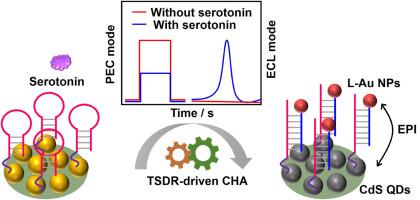
Combination of exciton-plasmon interaction and programmable DNA cyclic amplification for electrochemiluminescence/photoelectrochemical sensing of serotonin
A novel dual-mode electrochemiluminescence (ECL)/photoelectrochemistry (PEC) biosensor was developed for sensitive serotonin detection. In this system, the PEC signal was produced by CdS quantum dots (QDs), while the ECL signal originated from L-Au NPs (luminol decorated Au nanoparticles), thereby avoiding the external interference and signal fluctuations that typically arose from using the same materials for both signals. The presence of target serotonin initiated the non-enzymatic toehold-mediated strand displacement reaction (TSDR) on magnetic bead (MB), which was followed by catalytic hairpin assembly (CHA) on the sensing interface, leading to the aggregation of many L-Au NPs. The strong exciton-plasmon interactions (EPI) induced the energy transfer between CdS QDs and Au NPs, causing the significant suppression of the photocurrent. In addition, this design assured that the ECL and PEC respond in opposing manners and that no background ECL signal was detected, thereby greatly improving the sensitivity of the biosensor. Ultimately, the biosensor demonstrated a broad linear range from 5 pM to 1 μM with a detection limit of 1.6 pM, and also could be used for the assay of serum and urine samples with satisfactory results. With the advantages of high sensitivity, selectivity, accuracy and signal stability, this sensing strategy was helpful for disease diagnosis and the fundamental research of neurotransmitters.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Talanta
化学-分析化学
CiteScore
12.30
自引率
4.90%
发文量
861
审稿时长
29 days
期刊介绍:
Talanta provides a forum for the publication of original research papers, short communications, and critical reviews in all branches of pure and applied analytical chemistry. Papers are evaluated based on established guidelines, including the fundamental nature of the study, scientific novelty, substantial improvement or advantage over existing technology or methods, and demonstrated analytical applicability. Original research papers on fundamental studies, and on novel sensor and instrumentation developments, are encouraged. Novel or improved applications in areas such as clinical and biological chemistry, environmental analysis, geochemistry, materials science and engineering, and analytical platforms for omics development are welcome.
Analytical performance of methods should be determined, including interference and matrix effects, and methods should be validated by comparison with a standard method, or analysis of a certified reference material. Simple spiking recoveries may not be sufficient. The developed method should especially comprise information on selectivity, sensitivity, detection limits, accuracy, and reliability. However, applying official validation or robustness studies to a routine method or technique does not necessarily constitute novelty. Proper statistical treatment of the data should be provided. Relevant literature should be cited, including related publications by the authors, and authors should discuss how their proposed methodology compares with previously reported methods.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


