对“发现选择性蛋白激酶C-θ激酶抑制剂CC-90005”的更正
IF 6.8
1区 医学
Q1 CHEMISTRY, MEDICINAL
引用次数: 0
摘要
在图3和相关文本中,由于图准备中的印刷错误,Asp508被错误地标记为Asp409。更正后的图和更新后的文本行如下:图3。PKC-θ ATP口袋中3结合的对接模型(PDB ID 1XJD用于对接研究)。11889页:“碱性哌啶胺与侧链羧酸Asp465和催化残基Asp522形成两个盐桥,并与Asp508的主羰基形成第三个氢键。”这篇文章尚未被其他出版物引用。本文章由计算机程序翻译,如有差异,请以英文原文为准。
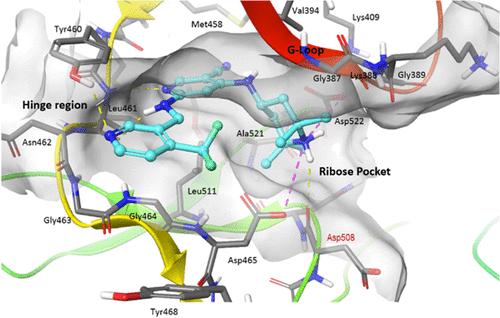
Correction to “Discovery of the Selective Protein Kinase C-θ Kinase Inhibitor, CC-90005”
In Figure 3 and the related text, Asp508 was incorrectly labeled as Asp409 due to typographical error in figure preparation. The corrected figure and updated line of text are as follow:
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Medicinal Chemistry
医学-医药化学
CiteScore
4.00
自引率
11.00%
发文量
804
审稿时长
1.9 months
期刊介绍:
The Journal of Medicinal Chemistry is a prestigious biweekly peer-reviewed publication that focuses on the multifaceted field of medicinal chemistry. Since its inception in 1959 as the Journal of Medicinal and Pharmaceutical Chemistry, it has evolved to become a cornerstone in the dissemination of research findings related to the design, synthesis, and development of therapeutic agents.
The Journal of Medicinal Chemistry is recognized for its significant impact in the scientific community, as evidenced by its 2022 impact factor of 7.3. This metric reflects the journal's influence and the importance of its content in shaping the future of drug discovery and development. The journal serves as a vital resource for chemists, pharmacologists, and other researchers interested in the molecular mechanisms of drug action and the optimization of therapeutic compounds.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


