空间分离双助催化剂修饰NH2-UiO-66构建人工光合组件增强光催化还原铀
IF 4.7
2区 化学
Q1 CHEMISTRY, INORGANIC & NUCLEAR
引用次数: 0
摘要
自然光合系统中光生电荷的快速迁移现象促使人们设计出能够快速分离电荷的高效光催化剂和高效反应动力学,用于光催化富集和分离铀废水中的铀U(VI)。在本研究中,我们开发了一种具有空间分离双助催化剂的仿生光催化体系MnOx/NH2-UiO-66-rGO (M/UiO-rGO)。其中,还原氧化石墨烯捕获电子参与还原反应,MnOx捕获空穴参与氧化反应。M/UiO-rGO催化剂在光催化还原铀方面表现出优异的性能(1 h内达到91.8%),即使在自然光条件下也表现出优异的铀去除率(80.4%)。利用多光谱耦合技术进一步证实了浓缩铀经历了一个“捕获-还原-自由基氧化-成核-结晶”的连续复杂反应过程。本研究为设计具有高效电荷分离和快速反应动力学的仿生光催化剂提供了一种可行的策略。本文章由计算机程序翻译,如有差异,请以英文原文为准。
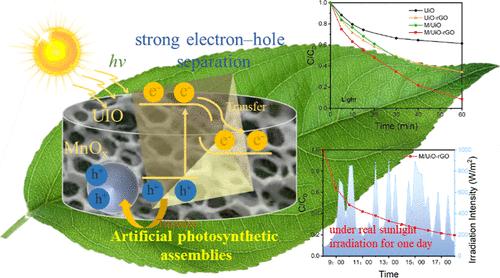
Artificial Photosynthetic Assemblies Constructed by NH2-UiO-66 Decorated with Spatially Separated Dual Cocatalysts for Enhanced Photocatalytic Uranium Reduction
The phenomenon of rapid migration of photogenerated charges in natural photosynthetic systems has motivated the design of efficient photocatalysts capable of fast charge separation and efficient reaction kinetics for photocatalytically assisted enrichment and separation of uranium U(VI) in uranium wastewater. In this study, we developed a biomimetic photocatalytic system MnOx/NH2-UiO-66-rGO (M/UiO-rGO) with spatially separated dual cocatalysts. Among them, rGO functions to capture electrons and participates in reduction reactions, while MnOx captures holes and participates in oxidation reactions. The M/UiO-rGO catalyst exhibits excellent performance in photocatalytic reduction of uranium (reaching 91.8% in 1 h), and even under natural light conditions, it exhibits excellent uranium removal ability (80.4%). Using multispectral coupling technology, we further confirmed that enriched uranium undergoes a continuous and complex reaction process of “capture-reduction-free radical oxidation–nucleation–crystallization”. This work presents a viable strategy for designing biomimetic photocatalysts with efficient charge separation and rapid reaction kinetics for environmental purification purposes.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Inorganic Chemistry
化学-无机化学与核化学
CiteScore
7.60
自引率
13.00%
发文量
1960
审稿时长
1.9 months
期刊介绍:
Inorganic Chemistry publishes fundamental studies in all phases of inorganic chemistry. Coverage includes experimental and theoretical reports on quantitative studies of structure and thermodynamics, kinetics, mechanisms of inorganic reactions, bioinorganic chemistry, and relevant aspects of organometallic chemistry, solid-state phenomena, and chemical bonding theory. Emphasis is placed on the synthesis, structure, thermodynamics, reactivity, spectroscopy, and bonding properties of significant new and known compounds.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


