RNA编辑策略在MECP2复制综合征小鼠模型和非人灵长类动物中拯救基因复制
IF 21.2
1区 医学
Q1 NEUROSCIENCES
引用次数: 0
摘要
甲基cpg结合蛋白2 (MECP2)基因的重复引起MECP2重复综合征(MDS)。为了使MDS中重复的MECP2正常化,我们开发了一种高保真的Cas13Y (hfCas13Y)系统,该系统能够靶向MECP2 (hfCas13Y- gmecp2)信使RNA,降解并降低人源化MECP2转基因小鼠大脑中的蛋白水平。此外,脑室腺相关病毒(AAV)在新生儿或成年MDS小鼠中递送hfCas13Y-gMECP2恢复了失调的基因表达并改善了行为缺陷。值得注意的是,用AAV9-hfCas13Y-gMECP2处理可将MECP2转基因小鼠的中位生存期从156.5天延长至226天。此外,对猴子的研究表明,单次注射AAV9-hfCas13Y-gMECP2足以在广泛的脑区中驱动hfCas13Y的稳健表达,MECP2的敲除效率达到52.19±0.03%,显著降低生物标志物基因GDF11的表达。我们的研究结果表明,靶向rna的hfCas13Y-gMECP2系统是一种有效的MDS干预手段,为治疗其他剂量敏感疾病提供了潜在的策略。本文章由计算机程序翻译,如有差异,请以英文原文为准。
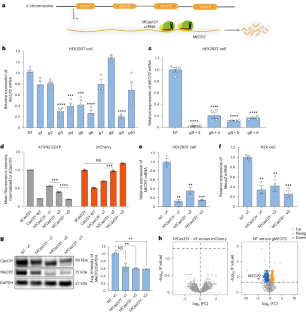

An RNA editing strategy rescues gene duplication in a mouse model of MECP2 duplication syndrome and nonhuman primates
Duplication of methyl-CpG-binding protein 2 (MECP2) gene causes MECP2 duplication syndrome (MDS). To normalize the duplicated MECP2 in MDS, we developed a high-fidelity Cas13Y (hfCas13Y) system capable of targeting the MECP2 (hfCas13Y-gMECP2) messenger RNA for degradation and reducing protein levels in the brain of humanized MECP2 transgenic mice. Moreover, the intracerebroventricular adeno-associated virus (AAV) delivery of hfCas13Y-gMECP2 in newborn or adult MDS mice restored dysregulated gene expression and improved behavior deficits. Notably, treatment with AAV9-hfCas13Y-gMECP2 extended the median survival of MECP2 transgenic mice from 156.5 to 226 d. Furthermore, studies with monkeys showed a single injection of AAV9-hfCas13Y-gMECP2 was sufficient to drive robust expression of hfCas13Y in widespread brain regions, with MECP2 knockdown efficiency reaching 52.19 ± 0.03% and significantly decreased expression of biomarker gene GDF11. Our results demonstrate that the RNA-targeting hfCas13Y-gMECP2 system is an effective intervention for MDS, providing a potential strategy for treating other dosage-sensitive diseases. Duplication of the MECP2 gene (encoding methyl-CpG-binding protein 2) causes MECP2 duplication syndrome. Here the authors develop a Cas13Y system capable of targeting the MECP2 mRNA for degradation and reducing protein levels in the brain of humanized MECP2-transgenic mice and nonhuman primates.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature neuroscience
医学-神经科学
CiteScore
38.60
自引率
1.20%
发文量
212
审稿时长
1 months
期刊介绍:
Nature Neuroscience, a multidisciplinary journal, publishes papers of the utmost quality and significance across all realms of neuroscience. The editors welcome contributions spanning molecular, cellular, systems, and cognitive neuroscience, along with psychophysics, computational modeling, and nervous system disorders. While no area is off-limits, studies offering fundamental insights into nervous system function receive priority.
The journal offers high visibility to both readers and authors, fostering interdisciplinary communication and accessibility to a broad audience. It maintains high standards of copy editing and production, rigorous peer review, rapid publication, and operates independently from academic societies and other vested interests.
In addition to primary research, Nature Neuroscience features news and views, reviews, editorials, commentaries, perspectives, book reviews, and correspondence, aiming to serve as the voice of the global neuroscience community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


