在Li10SiP2S12中共掺杂Sb和O以减少正交相含量,提高电化学性能
IF 6.3
2区 材料科学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
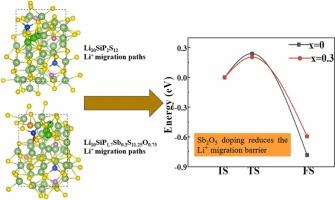
考虑到Li10SiP2S12 (LSiPS)固态电解质离子电导率高,电化学性能好,因此选择Li10SiP2S12 (LSiPS)电解质进行研究。然而,LSiPS电解质在合成过程中具有两相,通过掺杂和改性降低了其正交相含量,从而提高了物质的电化学性能。本文以Sb2O5为掺杂剂,合成了一系列改性硫化固体电解质。当Sb2O5掺杂量为x =0.3时,Li10SiP1.7Sb0.3S11.25O0.75 (LSiPSbSO)电解质的离子电导率可达1.18 mS cm-1。x射线衍射(XRD)测试证明,掺杂Sb2O5可以降低β-Li3PS4的正交相含量。密度泛函理论(DFT)计算表明,Sb和O的掺杂降低了Li+的迁移势垒,促进了Li+的扩散,提高了锂离子的电导率。LSiPSbSO电解液具有优异的电化学性能和良好的空气稳定性。组装后的对称锂电池在0.1 mA cm-2电流密度下可稳定循环622 h。用LSiPSbSO电解质组装的全固态电池的充放电实验证实了更高的比容量和循环稳定性。这项工作结合了系统的实验表征和充分的理论计算,证明了改性电解质具有优异的离子电导率和高空气稳定性,可以应用于全固态锂电池。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Sb and O co-doping in Li10SiP2S12 to reduce orthorhombic phase content and improve electrochemical performance
Li10SiP2S12 (LSiPS) electrolyte is chosen for the study considering that the solid-state electrolyte has high ionic conductivity along with good electrochemical performance. However, LSiPS electrolyte has two phases during the synthesis process, and the orthorhombic phase content is reduced by doping and modification of it thereby improving the electrochemical properties of the substance. In this work, a series of modified sulfide solid electrolytes are synthesized using Sb2O5 as dopant. The ionic conductivity of Li10SiP1.7Sb0.3S11.25O0.75 (LSiPSbSO) electrolyte can reach 1.18 mS cm-1 when the doping amount of Sb2O5 is x =0.3. X-ray diffraction (XRD) test proves that the doping of Sb2O5 can reduce the orthorhombic phase content of β-Li3PS4. Density functional theory (DFT) calculations show that the doping of Sb and O lowers the Li+ migration barrier, promotes the diffusion of Li+, and improves the conductivity of lithium ions. LSiPSbSO electrolyte has superior electrochemical performance as well as better air stability. The assembled lithium symmetric battery has a stable cycling process of 622 h at 0.1 mA cm-2 current density. Charge-discharge experiments of all-solid-state batteries assembled with LSiPSbSO electrolyte confirm higher specific capacity and cycling stability. This work combines systematic experimental characterization and sufficient theoretical calculations to demonstrate that the modified electrolyte has superior ionic conductivity and high air stability and can be applied to all-solid-state lithium batteries.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Alloys and Compounds
工程技术-材料科学:综合
CiteScore
11.10
自引率
14.50%
发文量
5146
审稿时长
67 days
期刊介绍:
The Journal of Alloys and Compounds is intended to serve as an international medium for the publication of work on solid materials comprising compounds as well as alloys. Its great strength lies in the diversity of discipline which it encompasses, drawing together results from materials science, solid-state chemistry and physics.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


