剖析人参的抗肥胖成分:人参多糖和人参皂苷如何针对肠道微生物群抑制高脂肪饮食引起的肥胖
IF 13
1区 综合性期刊
Q1 MULTIDISCIPLINARY SCIENCES
引用次数: 0
摘要
人参在治疗肥胖方面具有治疗潜力,实验和临床研究表明其抗肥胖作用是由肠道微生物群介导的。尽管如此,造成这种效应的具体化学成分在很大程度上仍未被确定。目的研究人参主要化学成分人参多糖(GP)和人参皂苷(GS)的抗肥胖作用及其机制,重点研究其对肠道菌群的影响。方法采用小鼠模型评价GP和GS对高脂饮食(HFD)诱导的肥胖的影响。通过化学分析、宏基因组学、RT-qPCR、ELISA和生化分析的结合来探索分子机制。结果甘油三酯或甘油三酯均能有效预防hfd喂养小鼠的肥胖,其作用机制与肠道菌群有关。化学分析显示GP和GS中存在不同的糖基。元基因组学数据显示,富含gp的菌种,如stercorirosorbacteroides和Clostridiales细菌,编码糖活性酶GH35、GH43和PL9_1,而富含gs的Sulfurospirillum halorespirans编码GH16_5。这些酶促进了GP和GS中糖基的利用,选择性地刺激细菌生长并重塑肠道微生物群。此外,被GP或GS富集的细菌种类编码了参与短链脂肪酸(SCFA)合成的特定功能基因(GP的K00625和K00925;K18118、K00100和K18122用于GS)和肠道糖异生(IGN) (K01678、K00024和K01596用于GP;K18118和K00278用于GS)。因此,GP和GS同时激活SCFA-GLP-1/PYY信号和IGN,以改善肥胖表型。结论甘油三酯和甘油三酯含有不同的糖基,可选择性刺激特定的肠道细菌,触发SCFA-GLP-1/PYY信号通路和IGN激活机制,从而降低hfd喂养小鼠的肥胖。该研究增强了对人参肠道微生物介导的抗肥胖作用的关键化学成分的理解。这种机制的理解为开发以人参为基础的药物或健康产品来对抗肥胖提供了有价值的见解。本文章由计算机程序翻译,如有差异,请以英文原文为准。
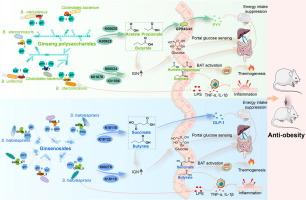
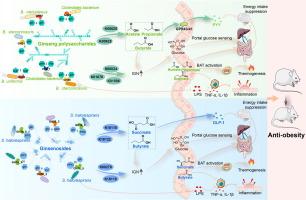
Dissecting the anti-obesity components of ginseng: How ginseng polysaccharides and ginsenosides target gut microbiota to suppress high-fat diet-induced obesity
Introduction
Ginseng demonstrates therapeutic potential in treating obesity, with both experimental and clinical studies suggesting its anti-obesity effects are mediated by gut microbiota. Nonetheless, the specific chemical components responsible for this effect remain largely unidentified.
Objectives
This study aims to investigate the anti-obesity effects and mechanisms of ginseng polysaccharides (GP) and ginsenosides (GS), the primary chemical components of ginseng, with a focus on their impact on gut microbiota.
Methods
The impact of GP and GS on high-fat diet (HFD)-induced obesity was assessed using a mouse model. Molecular mechanisms were explored through a combination of chemical analysis, metagenomics, RT-qPCR, ELISA, and biochemical assays.
Results
GP or GS administration effectively prevented adiposity in HFD-fed mice, and both effects were mediated by gut microbiota. Chemical analysis revealed diverse glycosyl groups in GP and GS. Metagenomics data suggested that GP-enriched species, e.g., Bacteroides stercorirosoris and Clostridiales bacterium encoded carbohydrate-active enzymes GH35, GH43 and PL9_1, while GS-enriched Sulfurospirillum halorespirans encoded GH16_5. These enzymes facilitated the utilization of glycosyl groups in GP and GS, selectively stimulating bacterial growth and reshaping the gut microbiota. Furthermore, bacterial species enriched by GP or GS encoded specific functional genes involved in short-chain fatty acid (SCFA) synthesis (K00625 and K00925 for GP; K18118, K00100, and K18122 for GS) and intestinal gluconeogenesis (IGN) (K01678, K00024, and K01596 for GP; K18118 and K00278 for GS). Consequently, the SCFA-GLP-1/PYY signaling and IGN were activated by both GP and GS to ameliorate obesity phenotypes.
Conclusion
GP and GS, containing diverse glycosyl groups, selectively stimulate specific gut bacteria, triggering mechanisms involved in SCFA-GLP-1/PYY signaling and IGN activation to reduce adiposity in HFD-fed mice. The study enhances understanding of the chemical components crucial for the gut microbiota-mediated anti-obesity effect of ginseng. The mechanistic understanding provides valuable insights for developing ginseng-based drugs or health products to combat obesity.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Advanced Research
Multidisciplinary-Multidisciplinary
CiteScore
21.60
自引率
0.90%
发文量
280
审稿时长
12 weeks
期刊介绍:
Journal of Advanced Research (J. Adv. Res.) is an applied/natural sciences, peer-reviewed journal that focuses on interdisciplinary research. The journal aims to contribute to applied research and knowledge worldwide through the publication of original and high-quality research articles in the fields of Medicine, Pharmaceutical Sciences, Dentistry, Physical Therapy, Veterinary Medicine, and Basic and Biological Sciences.
The following abstracting and indexing services cover the Journal of Advanced Research: PubMed/Medline, Essential Science Indicators, Web of Science, Scopus, PubMed Central, PubMed, Science Citation Index Expanded, Directory of Open Access Journals (DOAJ), and INSPEC.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


