VldE (Spr1875)是一种在活性位点具有四锌簇的肺炎球菌双态i,d-内肽酶
IF 13.1
1区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
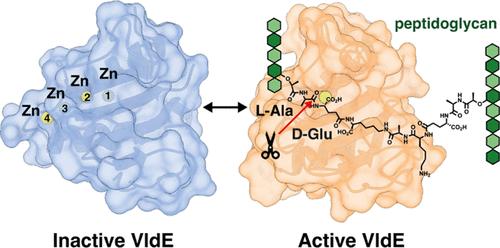
肺炎球菌细胞壁的重塑是由肽聚糖(PG)水解酶进行的,对于维持细菌细胞形状和确保生存是必要的,特别是在细胞分裂或应激反应期间。肺炎链球菌蛋白Spr1875在应激反应中发挥作用,两者均由VicRK双组分系统调节(类似于厚壁菌门中发现的WalRK TCS)。模块化的Spr1875在n端提供了一个假定的细胞壁结合模块和一个催化c端模块(Spr1875MT3),由一个长连接子连接。通过液相色谱/质谱分析,对全长蛋白和Spr1875MT3与基于PG的合成底物进行分析,发现Spr1875是一种l,d-内肽酶,更名为VldE (vicrk调节的l,d-内肽酶),可水解PG中的交联茎肽。值得注意的是,我们观察到不对称转换,特异性识别受体肽链。定位实验表明,该蛋白指向鼻中隔,这表明在应激条件下肺炎球菌的生长可能需要水解活性。我们的发现基于六个高分辨率x射线晶体学结构和分子动力学模拟,揭示了VldEMT3的两种状态。蛋白质在非催化状态和催化状态之间转换,非催化状态可以结合多达四个锌离子,因此充当Zn2+储层,催化状态可以与单个锌离子进行水解反应。此外,计算研究提供了对不对称交联PG的特定剪刀肽键的催化水活化和亲核攻击机制的见解。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Characterization of VldE (Spr1875), a Pneumococcal Two-State l,d-Endopeptidase with a Four-Zinc Cluster in the Active Site
Remodeling of the pneumococcal cell wall, carried out by peptidoglycan (PG) hydrolases, is imperative for maintaining bacterial cell shape and ensuring survival, particularly during cell division or stress response. The Streptococcus pneumoniae protein Spr1875 plays a role in stress response, both regulated by the VicRK two-component system (analogous to the WalRK TCS found in Firmicutes). Modular Spr1875 presents a putative cell-wall binding module at the N-terminus and a catalytic C-terminal module (Spr1875MT3) connected by a long linker. Assays of the full-length protein and Spr1875MT3 with PG-based synthetic substrates by liquid chromatography/mass spectrometry revealed Spr1875 as an l,d-endopeptidase, renamed VldE (for VicRK-regulated l,d-endopeptidase), which hydrolyzed the cross-linked stem peptide in the PG. Remarkably, we observed asymmetric turnover with specific recognition of the acceptor peptide strand. Localization experiments showed that the protein is directed to the septum, which suggests that muralytic activity could be required for pneumococcal growth under stress conditions. Our findings, based on six high-resolution X-ray crystallographic structures and molecular-dynamics simulations, reveal two states for VldEMT3. The protein transitions between a noncatalytic state that binds up to four zinc ions, thus behaving as a Zn2+ reservoir, and a catalytic state that performs the hydrolytic reaction with a single zinc ion. Furthermore, computational studies provide insight into the mechanism of catalytic-water activation and nucleophilic attack on the specific scissile peptide bond of the asymmetric cross-linked PG.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

ACS Catalysis
CHEMISTRY, PHYSICAL-
CiteScore
20.80
自引率
6.20%
发文量
1253
审稿时长
1.5 months
期刊介绍:
ACS Catalysis is an esteemed journal that publishes original research in the fields of heterogeneous catalysis, molecular catalysis, and biocatalysis. It offers broad coverage across diverse areas such as life sciences, organometallics and synthesis, photochemistry and electrochemistry, drug discovery and synthesis, materials science, environmental protection, polymer discovery and synthesis, and energy and fuels.
The scope of the journal is to showcase innovative work in various aspects of catalysis. This includes new reactions and novel synthetic approaches utilizing known catalysts, the discovery or modification of new catalysts, elucidation of catalytic mechanisms through cutting-edge investigations, practical enhancements of existing processes, as well as conceptual advances in the field. Contributions to ACS Catalysis can encompass both experimental and theoretical research focused on catalytic molecules, macromolecules, and materials that exhibit catalytic turnover.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


