机器学习辅助SERS揭示了表面修饰磁性纳米颗粒增强蛋白质分泌的生化特征
IF 8.2
2区 材料科学
Q1 MATERIALS SCIENCE, MULTIDISCIPLINARY
引用次数: 0
摘要
本研究通过蛋白质分泌和机器学习(ML)辅助表面增强拉曼散射(SERS)研究了Komagataella phaffii细胞与铁基磁性纳米颗粒(Fe3O4 MNPs)之间的相互作用。我们首次通过一锅共沉淀法制备了Fe3O4、Fe3O4@PEG、Fe3O4@PEI10kDa和Fe3O4@PEI25kDa MNPs。聚合物的加入对MNPs的形状、表面电荷、尺寸和尺寸分布有显著影响。聚乙烯亚胺(PEI)有效地完成了MNPs的表面改性,在NH4OH控制下MNPs的ζ电位值超过+25 mV。透射电子显微镜(TEM)显示,NH4OH合成的MNPs的均匀性更为明显。当我们使用250 ppm含铁的MNP溶液时,所有MNP都表现出优异的固定效率(>92%)。较小的MNPs均匀地包裹在菲氏k细胞表面,而较大的MNPs则呈不规则堆积。菲氏k细胞在所有MNP溶液中表现出优异的生存能力,最高可达1000ppm的铁浓度。最后,在Fe3O4@PEI10kDa mnp固定化细胞中获得了最高的重组azurin蛋白分泌率(约1.3倍)。ml辅助的SERS分析显示MNP与K. phaffii细胞的相互作用是由甘露蛋白和膜转运蛋白以及n -乙酰氨基葡萄糖(即几丁质)等蛋白质介导的。这些发现揭示了MNPs的大小和表面性质对菲氏k细胞固定化的影响,以及磁固定化对蛋白质分泌的巨大潜力。本文章由计算机程序翻译,如有差异,请以英文原文为准。
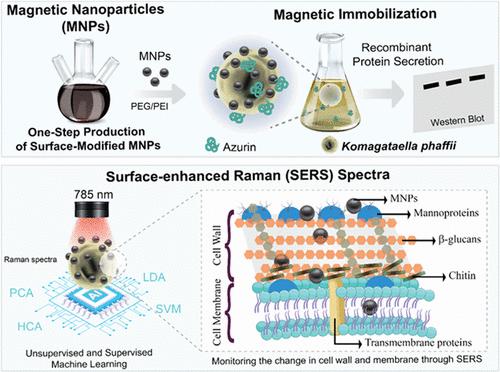
Machine Learning-Assisted SERS Reveals the Biochemical Signature of Enhanced Protein Secretion from Surface-Modified Magnetic Nanoparticles
This study introduces a novel investigation of the interaction between Komagataella phaffii cells and iron oxide-based magnetic nanoparticles (Fe3O4 MNPs) via protein secretion and machine learning (ML)-assisted surface-enhanced Raman scattering (SERS). For the first time, we produced Fe3O4, Fe3O4@PEG, Fe3O4@PEI10kDa, and Fe3O4@PEI25kDa MNPs by a one-pot coprecipitation reaction. The addition of polymers to the reaction conditions significantly affected the shape, surface charge, size, and size distribution of the MNPs. The surface modification of MNPs is effectively accomplished using polyethylenimine (PEI), and the ζ-potential values of the MNPs exceed +25 mV under the NH4OH control. The homogeneity of MNPs synthesized with NH4OH is more pronounced according to transmission electron microscopy (TEM) pictures. All MNPs exhibited excellent immobilization efficiency (>92%) when we used 250 ppm Fe-containing MNP solutions. Smaller MNPs uniformly encapsulated the surface of K. phaffii cells, whereas larger MNPs exhibited irregular accumulation. K. phaffii cells exhibited excellent viability in all MNP solutions at up to 1000 ppm of Fe concentrations. Finally, the highest recombinant azurin protein secretion rate was obtained in Fe3O4@PEI10kDa MNP-immobilized cells (about 1.3 times). The ML-assisted SERS analysis revealed that MNP interactions with K. phaffii cells were mediated by proteins such as mannoproteins and membrane transporter proteins as well as N-acetylglucosamine (i.e., chitin). These findings revealed the effect of the size and surface properties of MNPs on the immobilization of K. phaffii cells and the enormous potential of magnetic immobilization for protein secretion.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

ACS Applied Materials & Interfaces
工程技术-材料科学:综合
CiteScore
16.00
自引率
6.30%
发文量
4978
审稿时长
1.8 months
期刊介绍:
ACS Applied Materials & Interfaces is a leading interdisciplinary journal that brings together chemists, engineers, physicists, and biologists to explore the development and utilization of newly-discovered materials and interfacial processes for specific applications. Our journal has experienced remarkable growth since its establishment in 2009, both in terms of the number of articles published and the impact of the research showcased. We are proud to foster a truly global community, with the majority of published articles originating from outside the United States, reflecting the rapid growth of applied research worldwide.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


