缺乏电子的不对称Co中心与氧空位结合,NO3RR具有优异的活性和抗阴离子性能
IF 13.2
1区 工程技术
Q1 ENGINEERING, CHEMICAL
引用次数: 0
摘要
电催化还原废水中硝酸盐(NO3RR)合成无碳氨为实现氮循环提供了一个有前途的替代方案。co基电催化剂经常发生竞争性析氢反应,特别是在高反应电位下,导致NO3RR中法拉第效率低。本文通过对空心Co3O4前驱体进行原位氮处理,构建了富钴中心缺电子的不对称Co3O4纳米粒子(HP-Co)。这一过程触发了整个Co3O4的O晶格中N元素的合理超交换效应,产生缺电子的不对称Co中心和丰富的氧空位,使催化剂有利于硝酸盐离子的吸附和活化。实验结果表明,HP-Co的氨收率为59.7 ± 1.2 mg h−1 mgcat-1,最大法拉第效率为99 ± 0.5 %,硝酸盐转化率为95 ± 0.8 %,优于目前大多数催化剂。令人印象深刻的是,这种催化剂没有在NO3RR中积累中间NO2 -,排除了二次污染的威胁。近大气压NO气氛下的机理XPS研究证实,Co(II)和氧空位通过中间体的主导吸附和参与活化来决定反应过程。此外,理论计算结果表明,不对称的Co中心降低了NO*在反应位点上的中间反应的载流子。有趣的是,HP-Co通过高负电位对各种阴离子干扰表现出稳定的NO3RR性能,并具有出色的稳定性测试(60 h),进一步突出了其实际应用潜力。本文章由计算机程序翻译,如有差异,请以英文原文为准。
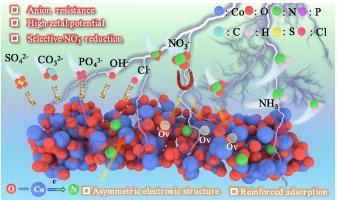
Electron-deficient asymmetric Co centers marry oxygen vacancy for NO3RR: Excellent activity and anion resistance property
The electrocatalytic reduction of nitrate (NO3RR) to carbon-free ammonia synthesis from wastewater offers a promising alternative for achieving nitrogen recycling. Co-based electrocatalysts often suffer from competitive hydrogen evolution reactions, especially at a high reaction potential resulting in low faradaic efficiency in NO3RR. Herein, we constructed electron-deficient asymmetric Co centers riching Co3O4 nanoparticles (HP-Co) through in-situ treatment of nitrogen for hollow Co3O4 precursor. Such a process triggers a rational superexchange effect of the N element in the O lattice of whole Co3O4, rendering electron-deficient asymmetric Co centers and abundant oxygen vacancies, making the catalyst conducive to the adsorption and activation of nitrate ions. Experimental results show an excellent ammonia yield of HP-Co of 59.7 ± 1.2 mg h−1 mgcat-1, with an exceptional maximum faradaic efficiency of 99 ± 0.5 % and a nitrate conversion rate of 95 ± 0.8 %, which outperforms most state-to-art catalysts. Impressively, such a catalyst showcases no accumulation of intermediate NO2– in the NO3RR excluding the threat of secondary pollution. The mechanistic XPS study with NO atmosphere at near-atmospheric pressure approved that the Co(II) and oxygen vacancy determine the reaction progress through dominant adsorption of intermediates and participated activation. Furthermore, the theoretical calculation results reveal that the asymmetric Co centers lower the reaction carrier of the reaction-determined step that NO* intermediates over reactive sites. Interestingly, HP-Co shows stable NO3RR performance against various anions interference by highly negative potential, coupling with excellent stability test (60 h), further highlighting its potential for practical applications.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Chemical Engineering Journal
工程技术-工程:化工
CiteScore
21.70
自引率
9.30%
发文量
6781
审稿时长
2.4 months
期刊介绍:
The Chemical Engineering Journal is an international research journal that invites contributions of original and novel fundamental research. It aims to provide an international platform for presenting original fundamental research, interpretative reviews, and discussions on new developments in chemical engineering. The journal welcomes papers that describe novel theory and its practical application, as well as those that demonstrate the transfer of techniques from other disciplines. It also welcomes reports on carefully conducted experimental work that is soundly interpreted. The main focus of the journal is on original and rigorous research results that have broad significance. The Catalysis section within the Chemical Engineering Journal focuses specifically on Experimental and Theoretical studies in the fields of heterogeneous catalysis, molecular catalysis, and biocatalysis. These studies have industrial impact on various sectors such as chemicals, energy, materials, foods, healthcare, and environmental protection.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


