现场自穿透纳米药物,实现双启动药物激活和由内而外的血栓消融
IF 16
1区 材料科学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
主要的常规抗血栓治疗方法往往存在治疗效果不理想和不良组织出血的风险。深血栓渗透,按需药物激活,以及在血栓内释放仍然是重大的挑战。虽然过去开发纳米药物和前药的努力以牺牲治疗效果为代价提高了安全性。在此,我们开发了一种自穿孔自激活的纳米组件,由氧化敏感的替格瑞洛(TGL)前药(TGL- s - fmoc, TSF)和IR808(光热/光动力双效光敏剂)组成。TSF很容易与IR808结合成一种无载体的混合纳米药物。在激光照射下,IR808使TSF光热溶栓和深凝块渗透,同时也协同促进由IR808产生的单线态氧(1O2)和凝块内内源性过氧化氢触发的前药活化。经过纤维蛋白靶向修饰后,纳米组件在体内具有良好的安全性,可实现自指示血栓靶向积累、自穿刺深血栓穿透、双重启动前药激活和由内到外的血栓消融。本研究促进了抗血栓前药和纳米药物的临床转化。本文章由计算机程序翻译,如有差异,请以英文原文为准。
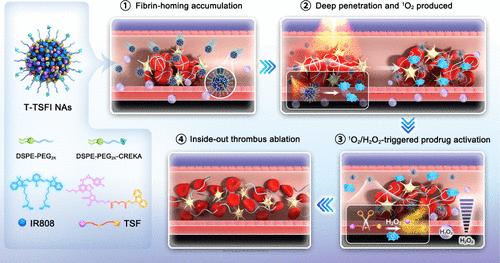
On-Site Self-Penetrating Nanomedicine Enabling Dual-Priming Drug Activation and Inside-Out Thrombus Ablation
Main conventional antithrombotic therapies often suffer from unsatisfactory treatment outcomes and the risk of undesirable tissue hemorrhage. Deep clot penetration, on-demand drug activation, and release within the clots remain significant challenges. While past efforts to develop nanomedicines and prodrugs have improved safety at the expense of therapeutic effects. Herein, we develop a self-piercing and self-activating nanoassembly composed of an oxidation-sensitive prodrug (TGL-S-Fmoc, TSF) of ticagrelor (TGL) and IR808 (a photothermal/photodynamic dual-effect photosensitizer). TSF readily coassembles with IR808 into a carrier-free hybrid nanomedicine. Upon laser irradiation, IR808 enables photothermal thrombolysis and deep clot penetration of TSF while also synergistically facilitating prodrug activation triggered by IR808-generated singlet oxygen (1O2) and the endogenous hydrogen peroxide within the clots. Following fibrin-targeting modification, the nanoassembly achieves self-indicating thrombus-targeted accumulation, self-piercing deep clot penetration, dual-priming prodrug activation, and inside-out thrombus ablation with favorable safety in vivo. This study advances the clinical translation of antithrombotic prodrugs and nanomedicines.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

ACS Nano
工程技术-材料科学:综合
CiteScore
26.00
自引率
4.10%
发文量
1627
审稿时长
1.7 months
期刊介绍:
ACS Nano, published monthly, serves as an international forum for comprehensive articles on nanoscience and nanotechnology research at the intersections of chemistry, biology, materials science, physics, and engineering. The journal fosters communication among scientists in these communities, facilitating collaboration, new research opportunities, and advancements through discoveries. ACS Nano covers synthesis, assembly, characterization, theory, and simulation of nanostructures, nanobiotechnology, nanofabrication, methods and tools for nanoscience and nanotechnology, and self- and directed-assembly. Alongside original research articles, it offers thorough reviews, perspectives on cutting-edge research, and discussions envisioning the future of nanoscience and nanotechnology.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


