溶液状态下花青素和蓝蛋白蓝铜中心的电子结构
IF 3.3
3区 化学
Q2 CHEMISTRY, INORGANIC & NUCLEAR
引用次数: 0
摘要
在铜L3边缘能区,测量了溶液状态下蓝铜蛋白(amicyanin和azurin)的x射线吸收近边结构(XANES)光谱。两种蛋白的吸收峰能量相当接近,但由于肩谱特征更明显,蓝蛋白的主边缘区域比花青素的更宽。从头算在全蛋白水平定性地再现了实验光谱。相对x射线吸收强度表明,活性位点铜-配体键的共价程度较蓝蛋白弱。本文章由计算机程序翻译,如有差异,请以英文原文为准。
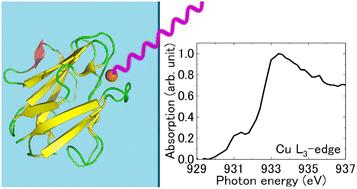
Electronic structures of blue copper centers of amicyanin and azurin in the solution state†
X-ray absorption near edge structure (XANES) spectra of blue copper proteins, amicyanin and azurin, in the solution state were measured in the copper L3-edge energy region. The absorption peak energies were quite similar for both proteins, while the main edge region for azurin was broader than that for amicyanin, owing to more pronounced shoulder spectral features in the former. Ab initio calculations at the whole protein level qualitatively reproduced the experimental spectra well. The relative X-ray absorption intensities suggest that the degree of covalency of the copper–ligand bond at the active site was weaker for amicyanin than that for azurin.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Dalton Transactions
化学-无机化学与核化学
CiteScore
6.60
自引率
7.50%
发文量
1832
审稿时长
1.5 months
期刊介绍:
Dalton Transactions is a journal for all areas of inorganic chemistry, which encompasses the organometallic, bioinorganic and materials chemistry of the elements, with applications including synthesis, catalysis, energy conversion/storage, electrical devices and medicine. Dalton Transactions welcomes high-quality, original submissions in all of these areas and more, where the advancement of knowledge in inorganic chemistry is significant.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


