基于网络药理学和实验研究探讨黄芪多糖对电离辐射心肌损伤的作用机制。
IF 4.7
2区 医学
Q2 IMMUNOLOGY
引用次数: 0
摘要
由于电离辐射不可避免地影响到位于纵隔附近的心脏,可发生不同程度的心肌损伤。因此,放疗在癌症治疗中的临床应用受到了极大的限制。然而,辐射诱发心脏病(RIHD)的分子机制尚不完全清楚,也缺乏针对该疾病的治疗策略。黄芪多糖(Astragalus polysaccharides, APS)是一种富含黄芪多糖的活性化合物。Bunge (AS)已被证明对各种心血管疾病具有心脏保护作用。因此,本研究旨在探讨黄芪多糖对RIHD的潜在保护作用及其潜在的分子机制。网络药理学结果表明,从AS作用于RIHD的有效组分的生物网络中鉴定出9个核心基因。氧化石墨烯富集分析结果表明,这些枢纽基因主要参与细胞凋亡、细胞增殖、炎症反应和对外界刺激的反应等生物学过程。KEGG富集分析结果显示,这些枢纽基因主要通过EGFR信号通路、PI3K/Akt信号通路、IL-17信号通路等途径调控RIHD的发生。在分子对接分析中,我们发现AKT1和mTOR与三种富含AS的糖苷具有良好而稳定的结合能力。体外和体内实验结果均表明,黄芪多糖不仅能改善RIHD大鼠心功能障碍、心肌损伤、炎症反应和心肌纤维化,还能减轻电离辐射刺激下H9C2细胞的凋亡和萎缩。此外,我们还发现APS改善了电离辐射诱导的自噬通量的积累,这可以通过逆转Beclin1, p62, LC3B蛋白和加速积累的自噬囊泡的降解来证实。雷帕霉素(Rapamycin, Rap)是一种经典的自噬通量诱导剂,可减弱APS对电离辐射刺激下H9C2细胞凋亡的促进作用。最后,我们在体外实验中发现,APS可以逆转电离辐射对PI3K/Akt/mTOR信号通路活性的抑制,从而改善电离辐射诱导的自噬通量积累、心肌细胞凋亡和萎缩。综上所述,本研究为理解自噬与凋亡相互作用的分子机制提供了重要证据,为APS联合自噬调节剂作为RIHD的治疗策略提供了新的方向和见解。本文章由计算机程序翻译,如有差异,请以英文原文为准。
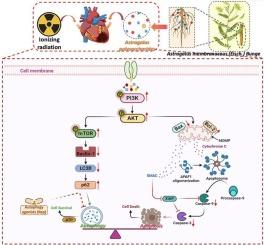
Elucidating the mechanism of action of astragalus polysaccharide on ionizing radiation-induced myocardial damage based on network pharmacology and experimental research
Due to the unavoidable impact of ionizing radiation on the heart located near the mediastinum, varying degrees of myocardial damage may occur. As a result, the clinical application of radiotherapy in cancer treatment is significantly limited. However, the molecular mechanisms underlying radiation-induced heart disease (RIHD) are not yet fully understood, and there is a lack of disease-specific treatment strategies. Astragalus polysaccharide (APS), is an active compound abundant in the traditional Chinese herb Astragalus membranaceus (Fisch.) Bunge (AS), has been shown to have cardioprotective effects against various cardiovascular diseases. Thus, this study aims to investigate the potential cardioprotective effect of APS on RIHD and its underlying molecular mechanisms. The network pharmacology results indicated that 9 core genes were identified from the biological network of the effective components of AS acting on RIHD. The results of GO enrichment analysis showed that these hub genes were mainly involved in biological processes such as cell apoptosis, cell proliferation, inflammatory response, and response to external stimuli. The results of KEGG enrichment analysis showed that these hub genes mainly regulated the occurrence of RIHD through pathways such as the EGFR signaling pathway, PI3K/Akt signaling pathway, IL-17 signaling pathway, and so on. In molecular docking analysis, we found that AKT1 and mTOR had good and stable binding abilities with the three types of glucosides rich in AS. The results of in vitro and in vivo experiments all showed that APS could not only improve cardiac dysfunction, myocardial injury, inflammatory response, and myocardial fibrosis in RIHD rats, but also alleviated apoptosis and atrophy of H9C2 cells under ionizing radiation stimulation. In addition, we also found that APS improved the accumulation of autophagic flux induced by ionizing radiation, which could be confirmed by the reversal of Beclin1, p62, LC3B proteins and accelerated degradation of accumulated autophagic vesicles. Rapamycin (Rap) was a classic autophagy flux inducer that could attenuate the improvement effect of APS on H9C2 cell apoptosis under ionizing radiation stimulation. Finally, we found that APS could reverse the inhibition of PI3K/Akt/mTOR signaling pathway activity by ionizing radiation in vitro, thereby improving ionizing radiation-induced autophagy flux accumulation, cardiomyocyte apoptosis, and atrophy. All in all, this study provides important evidence for understanding the molecular mechanisms of the cross-talk between autophagy and apoptosis, and provides new directions and insights for APS combined with autophagy regulators as a therapeutic strategy for RIHD.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
8.40
自引率
3.60%
发文量
935
审稿时长
53 days
期刊介绍:
International Immunopharmacology is the primary vehicle for the publication of original research papers pertinent to the overlapping areas of immunology, pharmacology, cytokine biology, immunotherapy, immunopathology and immunotoxicology. Review articles that encompass these subjects are also welcome.
The subject material appropriate for submission includes:
• Clinical studies employing immunotherapy of any type including the use of: bacterial and chemical agents; thymic hormones, interferon, lymphokines, etc., in transplantation and diseases such as cancer, immunodeficiency, chronic infection and allergic, inflammatory or autoimmune disorders.
• Studies on the mechanisms of action of these agents for specific parameters of immune competence as well as the overall clinical state.
• Pre-clinical animal studies and in vitro studies on mechanisms of action with immunopotentiators, immunomodulators, immunoadjuvants and other pharmacological agents active on cells participating in immune or allergic responses.
• Pharmacological compounds, microbial products and toxicological agents that affect the lymphoid system, and their mechanisms of action.
• Agents that activate genes or modify transcription and translation within the immune response.
• Substances activated, generated, or released through immunologic or related pathways that are pharmacologically active.
• Production, function and regulation of cytokines and their receptors.
• Classical pharmacological studies on the effects of chemokines and bioactive factors released during immunological reactions.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


