作为惰性键活化和还原不饱和体系的强效还原光催化剂的双乙醇酸盐
IF 19.1
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
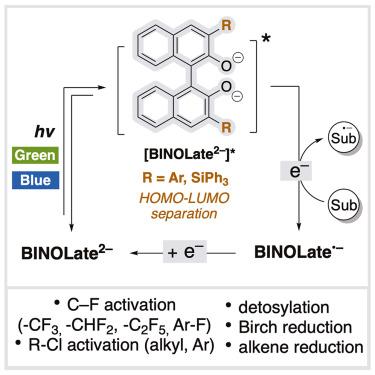
酚类化合物因其丰富和易于获取而越来越多地被用作光催化剂。然而,氧的高电负性和苯氧自由基的反应性阻碍了它们作为有效和广泛应用的还原性光氧化还原催化剂的潜力。在此,我们发现著名的1,1 ' -双-2-萘酚酸酯衍生物(BINOLates)是有效的还原性光催化剂。这些催化剂对惰性键的活化和不饱和键的还原是有效的,包括活化-CF3、-CF2H、-C2F5和芳酰氟的选择性CF活化,烷基和芳酰氯的活化,去甲基化,桦木还原和烯烃还原,显示出强大的还原能力和催化的多功能性。使用PhCF3作为限制试剂的脱氟烷基化进展顺利,这是报道的催化剂的一个具有挑战性的底物。即使在绿光下,双酚酸盐也可作为光氧化还原催化剂。这项工作为著名的双酚酸盐介绍了一种新的催化应用,表明了它们在光催化领域未来扩展应用的潜力。本文章由计算机程序翻译,如有差异,请以英文原文为准。
BINOLates as potent reducing photocatalysts for inert bond activation and reduction of unsaturated systems
Phenolates are increasingly studied as photocatalysts because of their abundance and easy accessibility. However, their potential as potent and broadly applicable reducing photoredox catalysts is hindered by the high electronegativity of oxygen and the reactivity of phenoxy radicals. Herein, we discovered that renowned 1,1′-Bi-2-naphtholate derivatives (BINOLates) are potent reducing photocatalysts. These catalysts are effective for the activation of inert bonds and the reduction of unsaturated bonds, including selective CF activation of activated –CF3,–CF2H, –C2F5, and aryl fluoride, activation of alkyl and aryl chlorides, detosylation, Birch reduction, and alkene reduction, demonstrating potent reducing ability and catalytic versatility. Defluoroalkylation using PhCF3 as a limiting reagent, a challenging substrate for reported catalysts, proceeded smoothly. BINOLates were applicable as photoredox catalysts even under green light. This work introduces a new catalytic application for the renowned BINOLates, suggesting the potential for future expansion of their applications in the realm of photocatalysis.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Chem
Environmental Science-Environmental Chemistry
CiteScore
32.40
自引率
1.30%
发文量
281
期刊介绍:
Chem, affiliated with Cell as its sister journal, serves as a platform for groundbreaking research and illustrates how fundamental inquiries in chemistry and its related fields can contribute to addressing future global challenges. It was established in 2016, and is currently edited by Robert Eagling.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


