水体系溶解力的分子机制
IF 9.6
1区 材料科学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
本文章由计算机程序翻译,如有差异,请以英文原文为准。
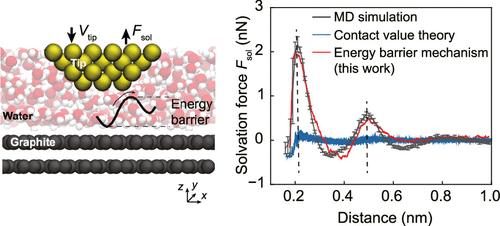
Molecular Mechanisms of Solvation Force for Aqueous Systems
Solvation force, stemming from the interfacial liquid structure, dominates the short-range interfacial interaction within a few nanometers across broad fields such as battery, lubrication, and colloid. However, achieving a quantitative understanding of solvation force for an aqueous system has remained elusive for decades, with the widely used contact value theory underestimating solvation force due to inherent assumptions. In this work, inspired by the flow field of liquid when two confining surfaces approach each other, we proposed a parameter-free expression for the solvation force acting on atomically smooth surfaces, quantitatively related to the energy barrier when liquid molecules are squeezed out from confinement. The effects of temperature and wetting properties of the surface on solvation force curves are found to be different. Solvation force measured by three-dimensional atomic force microscopy (3D-AFM) validates theoretical prediction on three types of surfaces ranging from hydrophilic to hydrophobic and reveals that the energy barrier is more intrinsic than density.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nano Letters
工程技术-材料科学:综合
CiteScore
16.80
自引率
2.80%
发文量
1182
审稿时长
1.4 months
期刊介绍:
Nano Letters serves as a dynamic platform for promptly disseminating original results in fundamental, applied, and emerging research across all facets of nanoscience and nanotechnology. A pivotal criterion for inclusion within Nano Letters is the convergence of at least two different areas or disciplines, ensuring a rich interdisciplinary scope. The journal is dedicated to fostering exploration in diverse areas, including:
- Experimental and theoretical findings on physical, chemical, and biological phenomena at the nanoscale
- Synthesis, characterization, and processing of organic, inorganic, polymer, and hybrid nanomaterials through physical, chemical, and biological methodologies
- Modeling and simulation of synthetic, assembly, and interaction processes
- Realization of integrated nanostructures and nano-engineered devices exhibiting advanced performance
- Applications of nanoscale materials in living and environmental systems
Nano Letters is committed to advancing and showcasing groundbreaking research that intersects various domains, fostering innovation and collaboration in the ever-evolving field of nanoscience and nanotechnology.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


