上皮钠通道中亚基依赖性功能调节的结构见解
IF 4.4
2区 生物学
Q2 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
上皮钠离子通道(ENaCs)在哺乳动物钠离子重吸收中起着至关重要的作用。迄今为止,已经确定了四个亚基-α, β, γ和δ -被认为形成不同的异质配合物。目前,只知道αβγ络合物的结构。为了研究具有不同亚基组成的通道的形成,并确定每个亚基如何贡献不同的通道特性,我们共同表达了人类的δ、β和γ。利用单粒子低温电子显微镜,我们观察到三种不同的ENaC复合物。该结构揭示了在不同配合物中β和γ位置保守,而α - β - γ三聚体中的α位置被δ或另一个β占据的模式。δ亚基诱导γ亚基的结构重排,这可能导致αβγ和δβγ通道活性的差异。这些结构变化提供了ENaC亚基组成如何调节通道功能的分子见解。本文章由计算机程序翻译,如有差异,请以英文原文为准。
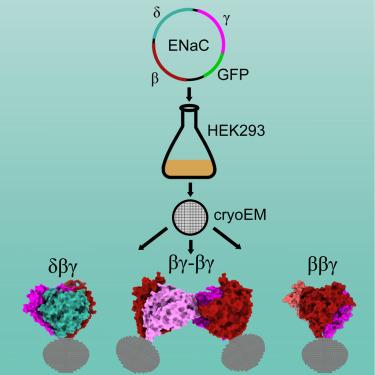
Structural insights into subunit-dependent functional regulation in epithelial sodium channels
Epithelial sodium channels (ENaCs) play a crucial role in Na+ reabsorption in mammals. To date, four subunits have been identified—α, β, γ, and δ—believed to form different heteromeric complexes. Currently, only the structure of the αβγ complex is known. To investigate the formation of channels with different subunit compositions and to determine how each subunit contributes to distinct channel properties, we co-expressed human δ, β, and γ. Using single-particle cryoelectron microscopy, we observed three distinct ENaC complexes. The structures unveil a pattern in which β and γ positions are conserved among the different complexes while the α position in αβγ trimer is occupied by either δ or another β. The δ subunit induces structural rearrangements in the γ subunit, which may contribute to the differences in channel activity between αβγ and δβγ channels. These structural changes provide molecular insights into how ENaC subunit composition modulates channel function.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Structure
生物-生化与分子生物学
CiteScore
8.90
自引率
1.80%
发文量
155
审稿时长
3-8 weeks
期刊介绍:
Structure aims to publish papers of exceptional interest in the field of structural biology. The journal strives to be essential reading for structural biologists, as well as biologists and biochemists that are interested in macromolecular structure and function. Structure strongly encourages the submission of manuscripts that present structural and molecular insights into biological function and mechanism. Other reports that address fundamental questions in structural biology, such as structure-based examinations of protein evolution, folding, and/or design, will also be considered. We will consider the application of any method, experimental or computational, at high or low resolution, to conduct structural investigations, as long as the method is appropriate for the biological, functional, and mechanistic question(s) being addressed. Likewise, reports describing single-molecule analysis of biological mechanisms are welcome.
In general, the editors encourage submission of experimental structural studies that are enriched by an analysis of structure-activity relationships and will not consider studies that solely report structural information unless the structure or analysis is of exceptional and broad interest. Studies reporting only homology models, de novo models, or molecular dynamics simulations are also discouraged unless the models are informed by or validated by novel experimental data; rationalization of a large body of existing experimental evidence and making testable predictions based on a model or simulation is often not considered sufficient.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


