钯位结构和相互转化对钯铜/沸石上乙烯瓦克氧化的影响
IF 6.5
1区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
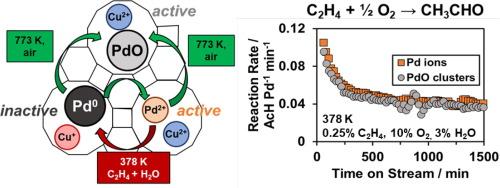
在PdCu/沸石上的非均相瓦克氧化可以取代腐蚀性的PdCu氯化物选择性氧化烯烃为羰基化合物。瓦克氧化通常被认为发生在Pd离子上,而PdO簇被认为是不活跃的。在这里,我们结合了钯离子滴定,x射线吸收光谱(XAS)和PdCu/Faujasite材料(1 wt% Pd, 4 wt% Cu, Si/Al 2.6)的反应动力学测量,以研究高温空气处理如何影响Pd位点的结构及其对乙烯瓦克氧化成乙醛的反应活性。通过NH4+离子反交换测定离子Pd的含量,并通过XAS验证。合成的催化剂只含有Pd2+离子,在高温(573 K, 773 K)的空气中煅烧后,Pd2+离子逐渐转化为小的PdO簇,从而产生一系列具有不同比例离子Pd的材料。瓦克速率(每Pd, 378 K, 3 kPa H2O)在这些材料中是不变的,这表明,与直觉相反,Pd离子和PdO簇是在本研究条件下瓦克氧化的相似活性位点前体。反应后的XAS证实,暴露在瓦克反应条件下,PdO簇和Pd离子之间没有明显的重组。我们进一步在还原和氧化瞬态进行原位XAS,以了解氧化还原活性Pd和Cu的比例。虽然大多数Pd2+和Cu2+离子在乙烯 + H2O中可还原为亚纳米级的Pd0簇和Cu+离子,但Pd0簇难以被O2(378 K)再氧化,这意味着需要快速的Pd再氧化以避免烧结。当催化剂在反应过程中或在还原条件下失活时,煅烧除去焦炭并再生一个Pd离子和PdO簇的活性池。这项工作为pdcu沸石上Wacker氧化的Pd活性位点及其稳定性提供了新的见解。本文章由计算机程序翻译,如有差异,请以英文原文为准。

Effects of Pd site structural changes on Wacker oxidation of ethylene over PdCu/zeolites
Heterogeneous Wacker oxidation over PdCu/zeolites could replace corrosive PdCu-chlorides for selective oxidation of olefins to carbonyl compounds. Wacker oxidation is traditionally thought to occur on Pd ions, while PdO clusters are assumed to be inactive. Here, we combined Pd ion titrations, X-ray absorption spectroscopy (XAS), and reaction kinetics measurements on a PdCu/Faujasite material (1 wt% Pd, 4 wt% Cu, Si/Al 2.6) to investigate how high-temperature air treatments impact the structure of Pd sites and their reactivity for Wacker oxidation of ethylene to acetaldehyde. The fraction of ionic Pd was assessed through NH4+-ion back-exchange corroborated by XAS. As-synthesized catalysts contain solely Pd2+ ions, which are progressively converted to small PdO clusters upon calcination in air at elevated temperature (573 K, 773 K), creating a series of materials with varying fractions of ionic Pd. Wacker rates (per Pd, 378 K, 3 kPa H2O) are invariant across these materials, showing, counterintuitively, that Pd ions and PdO clusters are similar active site precursors for Wacker oxidation at the conditions studied here. Post-reaction XAS confirms the absence of significant restructuring between PdO clusters and Pd ions following exposure to Wacker reaction conditions. We further performed in situ XAS during reduction and oxidation transients to understand the fraction of redox-active Pd and Cu. While most Pd2+ and Cu2+ ions are reducible in ethylene + H2O to sub-nanometer Pd0 clusters and Cu+ ions, Pd0 clusters are recalcitrant to re-oxidation by O2 (378 K), implying that rapid Pd re-oxidation is needed to avoid sintering. While catalysts deactivate during reaction or under reducing conditions, calcination removes coke and regenerates an active pool of Pd ions and PdO clusters. This work provides new insights into Pd active sites and their stability for Wacker oxidation over PdCu-zeolites.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Catalysis
工程技术-工程:化工
CiteScore
12.30
自引率
5.50%
发文量
447
审稿时长
31 days
期刊介绍:
The Journal of Catalysis publishes scholarly articles on both heterogeneous and homogeneous catalysis, covering a wide range of chemical transformations. These include various types of catalysis, such as those mediated by photons, plasmons, and electrons. The focus of the studies is to understand the relationship between catalytic function and the underlying chemical properties of surfaces and metal complexes.
The articles in the journal offer innovative concepts and explore the synthesis and kinetics of inorganic solids and homogeneous complexes. Furthermore, they discuss spectroscopic techniques for characterizing catalysts, investigate the interaction of probes and reacting species with catalysts, and employ theoretical methods.
The research presented in the journal should have direct relevance to the field of catalytic processes, addressing either fundamental aspects or applications of catalysis.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


