降低LiNi0.8Mn0.1Co0.1O2正极材料中的钴含量而不改变能量性能
IF 5.6
3区 材料科学
Q1 ELECTROCHEMISTRY
引用次数: 0
摘要
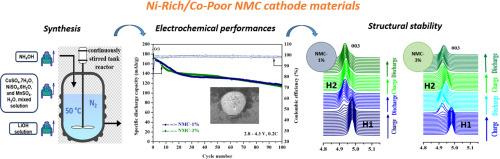
为了降低锂离子电池(lib)的成本和提高能量密度,使电动汽车(ev)实现长距离行驶,并在成本效益上与内燃机汽车竞争,开发富镍/贫钴层状阴极(LiNi1−x−yMnyCoxO2, x+y≤0.2)是当前最有效的策略之一。事实上,要增加容量(>;200 mAh.g-1)需要高的Ni含量,而低的Co含量有利于影响电池成本。因此,开发钴含量较低的正极新组合物对于提供替代方案至关重要。本研究是继前人对低钴NMC正极材料的研究之后,采用系统的方法,利用连续搅拌槽式反应器,采用共沉淀法合成了lini0.8 mn0.19 co0.010 o2 (NMC-1%)和lini0.8 mn0.17 co0.030 o2 (NMC-3%)两种富镍/贫钴正极材料。研究并比较了NMC-1%和NMC-3%阴极在0.1 C、0.2 C和0.5 C电流下的结构、形貌和电化学性能。此外,为了评估两种材料中Li+离子在脱嵌/嵌入过程中的结构演变,同步辐射操作衍射被用作理解第一次锂化/去锂化机制的有力工具。NMC-1%和NMC-3%阴极均表现出不同的电化学性能。在0.1℃时,它们的初始比放电容量为178 mAh。g-1和180毫安。g1分别;0.2℃,169 mAh。g-1和171 mAh.g-1;0.5℃时,138 mAh。g-1和156马赫,g-1。与我们之前研究的NMC-10%相比,NMC-1%在这些速率下实现了相当的容量保留,突出了其性能一致性。此外,两种材料在锂化过程中表现出不同的机制。本文章由计算机程序翻译,如有差异,请以英文原文为准。

Minimizing the cobalt content in LiNi0.8Mn0.1Co0.1O2 cathode material without altering the energetic performances
To reduce the cost and enhance the energy density of lithium-ion batteries (LIBs), enabling electric vehicles (EVs) to achieve long ranges and compete cost-effectively with vehicles with internal combustion engines, developing nickel-rich/cobalt-poor layered cathode (LiNi1−x−yMnyCoxO2, x + y ≤ 0.2) is one of the most current effective strategies. In fact, to increase the capacity (> 200 mAh.g-1) a high Ni content is required while to impact the battery cost a low Co content is favorable. Hence, the development of new compositions for the positive electrode with less cobalt content becomes essential to provide alternative options. This study is a follow of our previous investigation of low cobalt NMC cathode material, a systematic approach was adopted to synthesize two Ni-rich/Co-poor cathode materials, namely LiNi0.8Mn0.19Co0.01O2 (NMC-1 %) and LiNi0.8Mn0.17Co0.03O2 (NMC-3 %) via the co-precipitation method, utilizing a continuous stirred tank reactor. The structure, morphology, and electrochemical properties at 0.1 C, 0.2 C, and 0.5 C current rate, of NMC-1 % and NMC-3 % cathodes were investigated and compared. Furthermore, to assess the structural evolution throughout the de-intercalation/intercalation of Li+ ions in both materials, operando diffraction of synchrotron radiations was employed as powerful tool for understanding the first lithiation/ de-lithiation mechanism. Both NMC-1 % and NMC-3 % cathodes demonstrate distinct electrochemical performances. At 0.1 C, their initial specific discharge capacities are 178 mAh.g-1 and 180 mAh.g-1, respectively; at 0.2 C, 169 mAh.g-1 and 171 mAh.g-1; and at 0.5 C, 138 mAh.g-1 and 156 mAh.g-1. When compared with NMC-10 % from our previous study, NMC-1 % achieves comparable capacity retention at these rates, highlighting its performance consistency. Moreover, both materials exhibit distinct mechanisms upon the delithiation/lithiation process.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Electrochimica Acta
工程技术-电化学
CiteScore
11.30
自引率
6.10%
发文量
1634
审稿时长
41 days
期刊介绍:
Electrochimica Acta is an international journal. It is intended for the publication of both original work and reviews in the field of electrochemistry. Electrochemistry should be interpreted to mean any of the research fields covered by the Divisions of the International Society of Electrochemistry listed below, as well as emerging scientific domains covered by ISE New Topics Committee.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


