Fe(III)存在下Cr(III)光化学氧化成Cr(VI): Fe(III)浓度和UV波长的影响
IF 12.2
1区 环境科学与生态学
Q1 ENGINEERING, ENVIRONMENTAL
引用次数: 0
摘要
将六价铬还原成三价铬是降低环境毒性和流动性的关键,但对其反向过程的了解仍然较少。我们研究了在不同 pH 值和光波长(185、254 和 358 纳米)条件下,Fe(III) 存在下的 Cr(III) 氧化机制。在 358 纳米波长的光照下,pH 值为 3.0 时,Cr(VI) 的生成量在 11.65 μM 时达到峰值,这是由于光反应的 Fe(OH)²⁺ 产生了 -OH 自由基。虽然铁(III)通常会促进铬(III)的氧化,但浓度超过 0.5 mM 会抑制这一过程。由于铁(III)和水的光解作用,在 185 纳米波长的光下氧化作用最为强烈,最多可产生 217 μM 的六(Cr)。在 254 和 358 纳米波长下,-OH 完全由 Fe(III)光解产生,-OH 氧化 Fe(II),然后还原 Cr(VI),减缓了 Cr(III)的氧化。短波长、高能量的光可显著增强 Cr(III) 的氧化作用。在大气中的这种紫外线照射下,含铬(III)的气溶胶和颗粒可能会发生有害的转化,有可能通过酸性沉积物进入生态系统,对健康造成危害。本文章由计算机程序翻译,如有差异,请以英文原文为准。
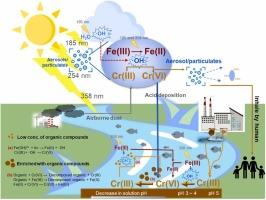
Photochemical oxidation of Cr(III) to Cr(VI) in the presence of Fe(III): Influence of Fe(III) concentration and UV wavelength
The reduction of Cr(VI) to Cr(III) is key to lowering environmental toxicity and mobility, but the reverse process remains less understood. We investigated Cr(III) oxidation mechanisms across various pH levels and light wavelengths (185, 254, and 358 nm) in the presence of Fe(III). At pH 3.0 under 358 nm light, Cr(VI) production peaked at 11.65 μM, driven by photo-reactive Fe(OH)²⁺ producing •OH radicals. While Fe(III) generally promotes Cr(III) oxidation, concentrations above 0.5 mM inhibited the process. Oxidation was most intense under 185 nm light, generating up to 217 μM of Cr(VI), due to Fe(III) and water photolysis. At 254 and 358 nm, •OH was solely produced by Fe(III) photolysis, where •OH oxidized Fe(II), which then reduced Cr(VI), slowing Cr(III) oxidation. Short-wavelength, high-energy light significantly enhances Cr(III) oxidation. Under such UV exposure in the atmosphere, Cr(III)-containing aerosols and particles may undergo harmful transformations, potentially entering ecosystems via acidic deposition and posing health risks.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Hazardous Materials
工程技术-工程:环境
CiteScore
25.40
自引率
5.90%
发文量
3059
审稿时长
58 days
期刊介绍:
The Journal of Hazardous Materials serves as a global platform for promoting cutting-edge research in the field of Environmental Science and Engineering. Our publication features a wide range of articles, including full-length research papers, review articles, and perspectives, with the aim of enhancing our understanding of the dangers and risks associated with various materials concerning public health and the environment. It is important to note that the term "environmental contaminants" refers specifically to substances that pose hazardous effects through contamination, while excluding those that do not have such impacts on the environment or human health. Moreover, we emphasize the distinction between wastes and hazardous materials in order to provide further clarity on the scope of the journal. We have a keen interest in exploring specific compounds and microbial agents that have adverse effects on the environment.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


