大柴胡煎剂治疗败血症的有效性和安全性:随机对照试验
IF 8.3
1区 医学
Q1 CHEMISTRY, MEDICINAL
引用次数: 0
摘要
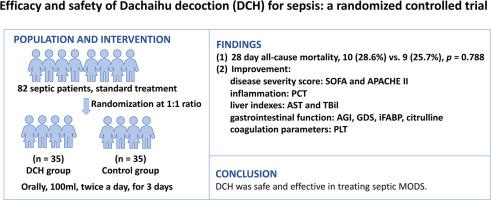
背景:脓毒症是一种以多器官功能障碍(MODS)为特征的危重疾病,提出了重大的治疗挑战。中药,特别是大柴胡汤,在解决败血症相关并发症方面显示出潜力。目的:综合评价DCH治疗脓毒症的疗效和安全性。方法:将符合条件的脓毒症患者按1:1的比例随机分为DCH组和对照组。干预疗程3 d。主要结局是28天全因死亡率、顺序器官衰竭评估(SOFA)和急性生理和慢性健康评估II (APACHE II)评分。次要结局通过各种临床参数进行评估,包括:(1)炎症标志物;(2)肝功能指标;(3)肾功能指标;(4)胃肠功能指标;(5)凝血参数。结果:70例脓毒症患者纳入完整分析集。对照组和DCH组28天全因死亡率无显著差异(10例(28.6%)比9例(25.7%),p = 0.788)。然而,DCH显著降低了SOFA评分(-3.0(四分位数范围,IQR, -5.5, -2.0) vs. 0.0 (IQR, -3.5, 0.0), APACHE II评分(-4.0 (IQR, -8.0, -2.0) vs. 0.0 (IQR, -2.0, 2.0), p < 0.001),天门氨酸转氨酶(-12.70 (IQR, -76.15, -3.35) vs. 2.40 (IQR, -8.05, 10.55), p = 0.001),总胆红素(-2.30 (IQR, -7.45, 3.62) vs. 1.80 (IQR, -2.35, 11.25), p = 0.028),急性胃肠道损伤等级(R¯,26.04 vs. 44.96, p < 0.001),胃肠道功能障碍评分(-5.0 (IQR, -6.0, -2.5) vs. -1.0 (IQR, -2.0, 0.5), p < 0.001),血清肠脂肪酸结合蛋白(-334.5 (IQR, -375.4, -288.8) vs. -34.8 (IQR, -104.2, -34.0), p < 0.001),瓜氨酸水平升高(6.97 (IQR, 6.76, 7.62) vs. 0.70 (IQR, -1.30, 2.83), p < 0.001),以及抑制血小板损失(-2.0 (IQR, -30.5, 45.5) vs. -26.0 (IQR, -65.0, 9.5), p = 0.043)。此外,DCH在炎症、肾功能和凝血方面均有改善,DCH组报告的严重不良事件较少(2.44% vs. 7.32%, p = 0.305)。结论:DCH治疗脓毒性MODS安全有效。尽管如此,需要进一步的研究来完善研究设计并提高脓毒症患者的预后。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Efficacy and safety of Dachaihu decoction for sepsis: A randomized controlled trial
Background
Sepsis is a critical condition characterized by multi-organ dysfunction (MODS) that presents significant treatment challenges. Traditional Chinese medicine (TCM), particularly Dachaihu decoction (DCH), has shown potential in addressing sepsis-related complications.
Purpose
To comprehensively evaluate the efficacy and safety of DCH in the treatment of sepsis.
Methods
Eligible septic patients were randomly assigned to either the DCH or control group in a 1:1 ratio. The intervention course lasted for 3 days. Primary outcomes were 28-day all-cause mortality, Sequential Organ Failure Assessment (SOFA) and Acute Physiology and Chronic Health Evaluation II (APACHE II) score. Secondary outcomes were assessed through various clinical parameters, including: (1) inflammatory markers; (2) liver function indices; (3) renal function markers; (4) gastrointestinal function metrics and (5) coagulation parameters.
Results
70 septic patients were included in the full analysis set. No significant difference in 28-day all-cause mortality was observed between the control and DCH groups (10 (28.6 %) vs. 9 (25.7 %), p = 0.788). However, DCH significantly reduced SOFA score (-3.0 (interquartile range, IQR, -5.5, -2.0) vs. 0.0 (IQR, -3.5, 0.0), p = 0.025), APACHE II score (-4.0 (IQR, -8.0, -2.0) vs. 0.0 (IQR, -2.0, 2.0), p < 0.001), aspartate transaminase (-12.70 (IQR, -76.15, -3.35) vs. 2.40 (IQR, -8.05, 10.55), p = 0.001), total bilirubin (-2.30 (IQR, -7.45, 3.62) vs. 1.80 (IQR, -2.35, 11.25), p = 0.028), acute gastrointestinal injury grade (, 26.04 vs. 44.96, p < 0.001), gastrointestinal dysfunction score (-5.0 (IQR, -6.0, -2.5) vs. -1.0 (IQR, -2.0, 0.5), p < 0.001), serum intestinal fatty acid-binding protein (-334.5 (IQR, -375.4, -288.8) vs. -34.8 (IQR, -104.2, -34.0), p < 0.001), and increased citrulline levels (6.97 (IQR, 6.76, 7.62) vs. 0.70 (IQR, -1.30, 2.83), p < 0.001), and also inhibiting platelet loss (-2.0 (IQR, -30.5, 45.5) vs. -26.0 (IQR, -65.0, 9.5), p = 0.043). Additionally, DCH demonstrated improvements in inflammation, renal function, and coagulation, with fewer serious adverse events reported in the DCH group (2.44 % vs. 7.32 %, p = 0.305).
Conclusion
DCH is both effective and safe in treating septic MODS. Nonetheless, further research is required to refine study designs and enhance outcomes for septic patients.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Phytomedicine
医学-药学
CiteScore
10.30
自引率
5.10%
发文量
670
审稿时长
91 days
期刊介绍:
Phytomedicine is a therapy-oriented journal that publishes innovative studies on the efficacy, safety, quality, and mechanisms of action of specified plant extracts, phytopharmaceuticals, and their isolated constituents. This includes clinical, pharmacological, pharmacokinetic, and toxicological studies of herbal medicinal products, preparations, and purified compounds with defined and consistent quality, ensuring reproducible pharmacological activity. Founded in 1994, Phytomedicine aims to focus and stimulate research in this field and establish internationally accepted scientific standards for pharmacological studies, proof of clinical efficacy, and safety of phytomedicines.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


