用于电化学硝酸盐还原和绿色合成氨的碳基催化剂综述
IF 9.2
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
电化学将硝酸盐(NO3−)转化为氨(NH3)是去除水体中NO3−污染以及生产农业所需NH3的一种很有前途的方法。硝酸电化学还原反应(NO3RR)以可再生电力为能源,运行条件温和,碳排放低,具有环境可持续性。本文综述了碳基电催化剂在NO3RR中的应用,包括纯碳材料、杂原子掺杂或金属键合催化剂以及碳基支撑复合材料。碳基电催化剂的制造方法、反应途径、机制、实验技术和理论都进行了批判性分析,并确定了工业环境中的实际限制。介绍了碳基电催化剂实现NO3RR,实现绿色氨清洁高效规模化生产的催化剂结构设计要点。本文章由计算机程序翻译,如有差异,请以英文原文为准。
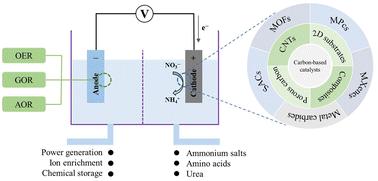
Review of carbon-based catalysts for electrochemical nitrate reduction and green ammonia synthesis
Electrochemical conversion of nitrate (NO3−) to ammonia (NH3) is a promising approach for removing NO3− contamination in water bodies as well as for producing NH3 necessary for agriculture. The electrochemical nitrate reduction reaction (NO3RR) uses renewable electricity as its energy source, operates under mild operating conditions, has low carbon emissions and is environmentally sustainable. Herein, we review the application of carbon-based electrocatalysts in NO3RR, including pure carbon materials, heteroatom doping or metal-bonding catalysts, and carbon substrate-supported composites. Fabrication methods, reaction pathways, mechanisms, experimental techniques and theory used in carbon-based electrocatalysts are critically analyzed and practical limitations are identified for industrial settings. Design aspects of catalyst structure are recommended for realizing NO3RR with carbon-based electrocatalysts and achieving clean and efficient large-scale production of green ammonia.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Green Chemistry
化学-化学综合
CiteScore
16.10
自引率
7.10%
发文量
677
审稿时长
1.4 months
期刊介绍:
Green Chemistry is a journal that provides a unique forum for the publication of innovative research on the development of alternative green and sustainable technologies. The scope of Green Chemistry is based on the definition proposed by Anastas and Warner (Green Chemistry: Theory and Practice, P T Anastas and J C Warner, Oxford University Press, Oxford, 1998), which defines green chemistry as the utilisation of a set of principles that reduces or eliminates the use or generation of hazardous substances in the design, manufacture and application of chemical products. Green Chemistry aims to reduce the environmental impact of the chemical enterprise by developing a technology base that is inherently non-toxic to living things and the environment. The journal welcomes submissions on all aspects of research relating to this endeavor and publishes original and significant cutting-edge research that is likely to be of wide general appeal. For a work to be published, it must present a significant advance in green chemistry, including a comparison with existing methods and a demonstration of advantages over those methods.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


