离子对微溶胶团簇太赫兹光谱的影响
IF 4.8
2区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
含有Na+和Cl -离子的水团簇在海盐气溶胶的大气化学中起着关键作用。虽然Na+显然深埋在内部,但Cl -似乎是一个变色龙,因为有证据表明表面局部化和内部溶剂化状态。因此,揭示Cl -在团簇中的首选位置仍然具有挑战性。在这里,我们研究了太赫兹光谱,一个直接探测水中氢键的强大工具,是否提供了Cl -离子在水簇中的位置的见解。我们对含有单个Cl -离子和多达64个水分子的水团簇进行了从头开始的分子动力学模拟,以计算参考Na+和体积的太赫兹光谱。64-水Cl -团簇的太赫兹谱与体溶液的太赫兹谱基本一致。令人惊讶的是,这种匹配并不是象现象学线形分析所表明的那样,是由Cl -的块状溶剂化引起的。相反,这种相似性源于Cl -主要位于簇表面,从而使水-水相互作用在很大程度上不受干扰。本文章由计算机程序翻译,如有差异,请以英文原文为准。
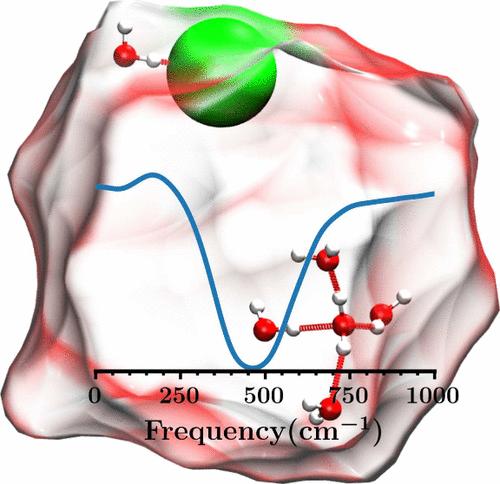
Ion Effects on Terahertz Spectra of Microsolvated Clusters
Water clusters containing Na+ and Cl– ions play a key role in the atmospheric chemistry of sea salt aerosols. While Na+ is clearly buried deep inside, Cl– appears to be a chameleon since evidence for both surface-localized and interior solvation states are reported. Thus, disclosing the preferred location of Cl– within clusters remains challenging. Here, we investigate whether THz spectroscopy, a powerful tool for directly probing hydrogen bonds in water, provides insights into the location of Cl– ions in water clusters. We performed ab initio molecular dynamics simulations on water clusters containing a single Cl– ion and up to 64 water molecules to compute the THz spectra with reference to Na+ and bulk. The THz spectrum of the 64-water Cl– cluster closely agrees with that of the bulk solution. Surprisingly, this match is not caused by bulk-like solvation of Cl– as suggested by phenomenological line shape analyses. Instead, the similarity stems from Cl– being mostly located at the cluster surface, thus leaving the water–water interactions largely unperturbed.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

The Journal of Physical Chemistry Letters
CHEMISTRY, PHYSICAL-NANOSCIENCE & NANOTECHNOLOGY
CiteScore
9.60
自引率
7.00%
发文量
1519
审稿时长
1.6 months
期刊介绍:
The Journal of Physical Chemistry (JPC) Letters is devoted to reporting new and original experimental and theoretical basic research of interest to physical chemists, biophysical chemists, chemical physicists, physicists, material scientists, and engineers. An important criterion for acceptance is that the paper reports a significant scientific advance and/or physical insight such that rapid publication is essential. Two issues of JPC Letters are published each month.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


