利用小角 X 射线/中子散射提取溶液中蛋白质间的方向和距离相关相互作用位势
IF 4.8
2区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
本文章由计算机程序翻译,如有差异,请以英文原文为准。
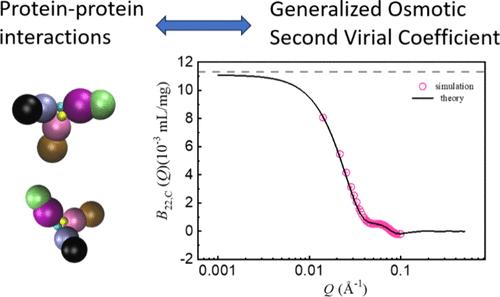
Extracting Orientation and Distance-Dependent Interaction Potentials between Proteins in Solutions Using Small-Angle X-ray/Neutron Scattering
Nonspecific protein–protein interactions (PPIs) are key to understanding the behavior of proteins in solutions. However, experimentally measuring anisotropic PPIs as a function of orientation and distance has been challenging. Here, we propose to measure a new parameter, the generalized second virial coefficient, B22(Q), to address this challenge. B22(Q) can be measured by using small-angle X-ray/neutron scattering (SAXS/SANS) at finite Q values, where Q is the magnitude of the scattering wave vector. We develop the analytical theory here to calculate B22(Q) with any known interprotein potentials including anisotropic interaction potentials. This method overcomes the challenges and limitations of commonly used methods for extracting PPI information, namely, using integral approximations to solve the Ornstein–Zernike equation by fitting SAXS/SANS data. The accuracy of this analytical theory is further evaluated with computer simulations using a model system. Not only can our method greatly extend the capability of SAXS/SANS to investigate PPIs of many proteins, but it is also applicable to a wide variety of colloidal systems where anisotropic interaction potentials are important.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

The Journal of Physical Chemistry Letters
CHEMISTRY, PHYSICAL-NANOSCIENCE & NANOTECHNOLOGY
CiteScore
9.60
自引率
7.00%
发文量
1519
审稿时长
1.6 months
期刊介绍:
The Journal of Physical Chemistry (JPC) Letters is devoted to reporting new and original experimental and theoretical basic research of interest to physical chemists, biophysical chemists, chemical physicists, physicists, material scientists, and engineers. An important criterion for acceptance is that the paper reports a significant scientific advance and/or physical insight such that rapid publication is essential. Two issues of JPC Letters are published each month.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


