β-酮酰基吡唑作为2C合子在邻羟基查尔酮不对称环化中的应用:反式3,4-二氢香豆素的立体选择性构造
IF 3.6
2区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
摘要
在双功能方酰胺叔胺的催化下,建立了β-酮酰基吡唑与邻羟基查尔酮的不对称串联酯化/迈克尔加成反应。在温和的反应条件下,通常以高收率(高达93%)获得各种生物相关的3,4-二氢香豆素衍生物,具有优异的非映体和对映体选择性(>19:1 dr,高达93% ee)。该反应代表了β-酮酰基吡唑在催化不对称环化中作为2C构建单元的成功应用。本文章由计算机程序翻译,如有差异,请以英文原文为准。
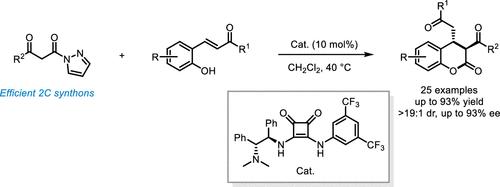
Application of β-Keto Acylpyrazoles as 2C Synthons in Asymmetric Cyclizations of ortho-Hydroxychalcones: Stereoselective Construction of trans-3,4-Dihydrocoumarins
An asymmetric tandem esterification/Michael addition reaction of β-keto acylpyrazoles with o-hydroxychalcones has been established under the catalysis of a bifunctional squaramide-tertiary amine. A wide variety of biorelevant 3,4-dihydrocoumarin derivatives were generally obtained in high yields (up to 93%) with excellent diastereo- and enantioselectivities (>19:1 dr, up to 93% ee) under mild reaction conditions. This reaction represents the successful application of β-keto acylpyrazoles as 2C building blocks in catalytic asymmetric cyclizations.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Organic Chemistry
化学-有机化学
CiteScore
6.20
自引率
11.10%
发文量
1467
审稿时长
2 months
期刊介绍:
Journal of Organic Chemistry welcomes original contributions of fundamental research in all branches of the theory and practice of organic chemistry. In selecting manuscripts for publication, the editors place emphasis on the quality and novelty of the work, as well as the breadth of interest to the organic chemistry community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


