引入二硫键稳定抗体轻链交换结构的实验与计算研究
IF 6.8
1区 医学
Q1 CHEMISTRY, MEDICINAL
引用次数: 0
摘要
不同平台的开发将有助于设计功能性抗体,以提高基于抗体的药物的效率。抗体轻链#4C214A可变区可能发生三维结构域交换(3D-DS),一对交换结构域的二聚体可能相互作用形成四聚体。在本研究中,为了稳定#4C214A的3D-DS二聚体结构,将A链上的Val2(交换区)和G链上的Thr97替换为Cys残基,生成#4 V2C/T97C/C214A,其中Cys2-Cys97二硫键交联不同原聚体的A链和G链。#4 V2C/T97C/C214A四聚体在低蛋白浓度(6 μM)下不解离成单体;然而,一些四聚体通过二硫键还原转化为单体。通过分子动力学模拟对两种3D-DS二聚体的四聚化进行了二维自由能谱分析。这些结果表明,引入二硫键有助于通过3D-DS控制可变区域的二聚化/解离。本文章由计算机程序翻译,如有差异,请以英文原文为准。
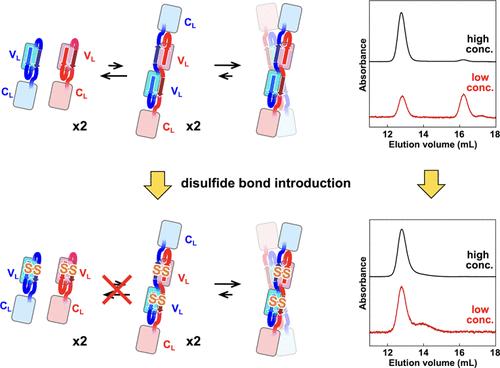
Experimental and Computational Studies on Domain-Swapped Structure Stabilization of an Antibody Light Chain by Disulfide Bond Introduction
Development of different platforms would be useful for designing functional antibodies to improve the efficiency of antibody-based drugs. Three-dimensional domain swapping (3D-DS) may occur in the variable region of antibody light chain #4C214A, and a pair of domain-swapped dimers may interact with each other to form a tetramer. In this study, to stabilize the 3D-DS dimer structure in #4C214A, Val2 in strand A (swapping region) and Thr97 in strand G were replaced with Cys residues, generating #4 V2C/T97C/C214A with a Cys2–Cys97 disulfide bond that cross-links strands A and G of different protomers. The #4 V2C/T97C/C214A tetramer did not dissociate into monomers at low protein concentration (6 μM); however, some of the tetramers were converted to monomers by disulfide bond reduction. Two-dimensional free energy profile analysis for the tetramerization of two 3D-DS dimers was performed by molecular dynamics simulation. These results show that disulfide bond introduction is useful for controlling the dimerization/dissociation of the variable region through 3D-DS.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Medicinal Chemistry
医学-医药化学
CiteScore
4.00
自引率
11.00%
发文量
804
审稿时长
1.9 months
期刊介绍:
The Journal of Medicinal Chemistry is a prestigious biweekly peer-reviewed publication that focuses on the multifaceted field of medicinal chemistry. Since its inception in 1959 as the Journal of Medicinal and Pharmaceutical Chemistry, it has evolved to become a cornerstone in the dissemination of research findings related to the design, synthesis, and development of therapeutic agents.
The Journal of Medicinal Chemistry is recognized for its significant impact in the scientific community, as evidenced by its 2022 impact factor of 7.3. This metric reflects the journal's influence and the importance of its content in shaping the future of drug discovery and development. The journal serves as a vital resource for chemists, pharmacologists, and other researchers interested in the molecular mechanisms of drug action and the optimization of therapeutic compounds.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


