以 EphA4 配体结合域为目标的受约束β-发夹
IF 6.8
1区 医学
Q1 CHEMISTRY, MEDICINAL
引用次数: 0
摘要
本文章由计算机程序翻译,如有差异,请以英文原文为准。
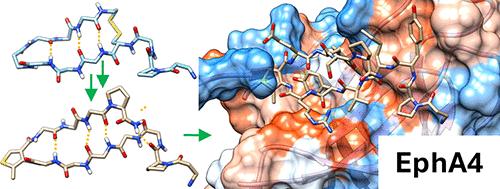
Constrained β-Hairpins Targeting the EphA4 Ligand Binding Domain
The activity of the receptor tyrosine kinase EphA4 has been implicated in several pathologies including oncology (gastric and pancreatic cancers) and neurodegenerative diseases (amyotrophic lateral sclerosis and Alzheimer’s disease). However, advances in validating EphA4 as a possible drug target have been limited by the lack of suitable pharmacological inhibitors. Recently, we reported on the design of potent EphA4 agonistic agents targeting its ligand binding domain (LBD). Based on previous studies with a phage display cyclic peptide inhibitor, we designed a β-hairpin mimetic with high affinity for EphA4-LBD. These agents hold great promise for further validation and development of EphA4-based therapeutics. Moreover, our studies introduce a possible strategy for the design of constrained β-hairpin peptides.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Medicinal Chemistry
医学-医药化学
CiteScore
4.00
自引率
11.00%
发文量
804
审稿时长
1.9 months
期刊介绍:
The Journal of Medicinal Chemistry is a prestigious biweekly peer-reviewed publication that focuses on the multifaceted field of medicinal chemistry. Since its inception in 1959 as the Journal of Medicinal and Pharmaceutical Chemistry, it has evolved to become a cornerstone in the dissemination of research findings related to the design, synthesis, and development of therapeutic agents.
The Journal of Medicinal Chemistry is recognized for its significant impact in the scientific community, as evidenced by its 2022 impact factor of 7.3. This metric reflects the journal's influence and the importance of its content in shaping the future of drug discovery and development. The journal serves as a vital resource for chemists, pharmacologists, and other researchers interested in the molecular mechanisms of drug action and the optimization of therapeutic compounds.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


