基于复合生物活性离子的仿生细胞外囊泡用于治疗缺血性骨病
IF 15.8
1区 材料科学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
本文章由计算机程序翻译,如有差异,请以英文原文为准。
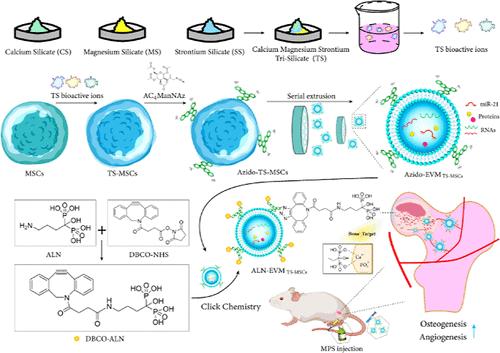
Biomimetic Extracellular Vesicles Based on Composite Bioactive Ions for the Treatment of Ischemic Bone Disease
Extracellular vesicles (EVs) have demonstrated considerable potential in the treatment of ischemic bone diseases, such as glucocorticoid-induced osteonecrosis of the femoral head (GIONFH). However, the clinical application of EVs faces challenges such as low yield, poor bioactivity, and lack of targeting. Herein, we have developed a platform of multiengineered extracellular vesicle mimetics (EVMs) to address these challenges. By stimulating mesenchymal stem cells (MSCs) with multibioactive ions from TS (Trisilicate, a mixture of calcium silicate, magnesium silicate, and strontium silicate), we obtained endogenously modified TS-MSCs. From these, we further prepared a large quantity of bioactive EVMTS-MSCs through a straightforward extrusion method. Moreover, by integrating metabolic glycoengineering with click chemistry strategies, alendronate (ALN) was surface-modified on EVMTS-MSCs to further prepare ALN-EVMTS-MSCs. The engineered ALN-EVMTS-MSCs demonstrated bone-targeting effects, promoting osteogenesis and angiogenesis. This promoting effect is attributed to the rich presence of miR-21 in the TS-modified EVM, which further silences PTEN to activate the PI3K/AKT signaling pathway, thereby enhancing osteogenesis and angiogenesis. Our treatment strategy for ischemic bone diseases is based on a multiengineered, biomaterial-inspired, metabolic glycoengineering, and click chemistry-based platform of EVM. This study also provides an enhanced understanding of the development and application of engineered vesicles in disease treatment.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

ACS Nano
工程技术-材料科学:综合
CiteScore
26.00
自引率
4.10%
发文量
1627
审稿时长
1.7 months
期刊介绍:
ACS Nano, published monthly, serves as an international forum for comprehensive articles on nanoscience and nanotechnology research at the intersections of chemistry, biology, materials science, physics, and engineering. The journal fosters communication among scientists in these communities, facilitating collaboration, new research opportunities, and advancements through discoveries. ACS Nano covers synthesis, assembly, characterization, theory, and simulation of nanostructures, nanobiotechnology, nanofabrication, methods and tools for nanoscience and nanotechnology, and self- and directed-assembly. Alongside original research articles, it offers thorough reviews, perspectives on cutting-edge research, and discussions envisioning the future of nanoscience and nanotechnology.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


