空间精确和微创递送多肽到脊髓的行为调节
IF 16
1区 材料科学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
血脊髓屏障(BSCB)严格调节分子从血液到脊髓的运输。在此,我们提出了一种高空间分辨率的BSCB通透性瞬态调节和多肽局部递送到脊髓的行为调节方法。这种方法利用光学刺激血管靶向纳米颗粒,并允许将bscb不渗透分子输送到脊髓中,而不会显著激活胶质细胞或影响动物的运动行为。我们展示了在腰椎区域使用光纤和BSCB渗透性调制的微创光输送到脊髓。我们的BSCB调节方法允许将bombesin(一种中枢作用的瘙痒诱导肽)递送到脊髓中,并诱导小鼠瘙痒行为的快速和短暂增加。这种微创方法可以在没有基因修饰的情况下进行行为调节,并且有望将广泛的生物制剂输送到脊髓中,用于高时空分辨率的潜在治疗。本文章由计算机程序翻译,如有差异,请以英文原文为准。
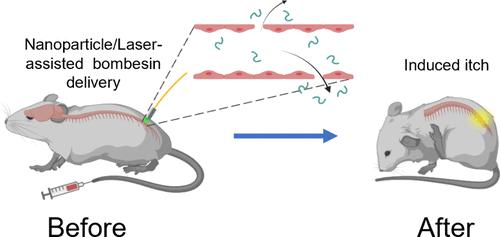
Spatially Precise and Minimally Invasive Delivery of Peptides to the Spinal Cord for Behavior Modulation
The blood–spinal cord barrier (BSCB) tightly regulates the transport of molecules from the blood to the spinal cord. Herein, we present an approach for transient modulation of BSCB permeability and localized delivery of peptides into the spinal cord for behavior modulation with high spatial resolution. This approach utilizes optical stimulation of vasculature-targeted nanoparticles and allows delivery of BSCB-impermeable molecules into the spinal cord without significant glial activation or impact on animal locomotor behavior. We demonstrate minimally invasive light delivery into the spinal cord using an optical fiber and BSCB permeability modulation in the lumbar region. Our method of BSCB modulation allows the delivery of bombesin, a centrally acting and itch-inducing peptide, into the spinal cord and induces a rapid and transient increase in itching behaviors in mice. This minimally invasive approach enables behavior modulation without genetic modifications and is promising for delivering a wide range of biologics into the spinal cord for potential therapy with high spatiotemporal resolution.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

ACS Nano
工程技术-材料科学:综合
CiteScore
26.00
自引率
4.10%
发文量
1627
审稿时长
1.7 months
期刊介绍:
ACS Nano, published monthly, serves as an international forum for comprehensive articles on nanoscience and nanotechnology research at the intersections of chemistry, biology, materials science, physics, and engineering. The journal fosters communication among scientists in these communities, facilitating collaboration, new research opportunities, and advancements through discoveries. ACS Nano covers synthesis, assembly, characterization, theory, and simulation of nanostructures, nanobiotechnology, nanofabrication, methods and tools for nanoscience and nanotechnology, and self- and directed-assembly. Alongside original research articles, it offers thorough reviews, perspectives on cutting-edge research, and discussions envisioning the future of nanoscience and nanotechnology.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


