三级淋巴结构中的皮肤免疫-间质相互作用促进了化脓性扁桃体炎的自身免疫发病机制
IF 25.5
1区 医学
Q1 IMMUNOLOGY
引用次数: 0
摘要
本文章由计算机程序翻译,如有差异,请以英文原文为准。
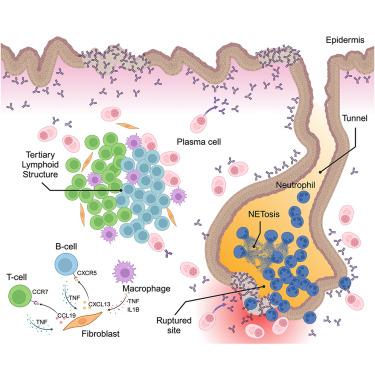
Skin immune-mesenchymal interplay within tertiarylymphoid structures promotes autoimmunepathogenesis in hidradenitis suppurativa
Hidradenitis suppurativa (HS) is a chronic, debilitating inflammatory skin disease characterized by keratinized epithelial tunnels that grow deeply into the dermis. Here, we examined the immune microenvironment within human HS lesions. Multi-omics profiling and multiplexed imaging identified tertiary lymphoid structures (TLSs) near HS tunnels. These TLSs were enriched with proliferative T cells, including follicular helper (Tfh), regulatory (Treg), and pathogenic T cells (IL17A+ and IFNG+), alongside extensive clonal expansion of plasma cells producing antibodies reactive to keratinocytes. HS fibroblasts express CXCL13 or CCL19 in response to immune cytokines. Using a microfluidic system to mimic TLS on a chip, we found that HS fibroblasts critically orchestrated lymphocyte aggregation via tumor necrosis factor alpha (TNF-α)-CXCL13 and TNF-α-CCL19 feedback loops with B and T cells, respectively; early TNF-α blockade suppressed aggregate initiation. Our findings provide insights into TLS formation in the skin, suggest therapeutic avenues for HS, and reveal mechanisms that may apply to other autoimmune settings, including Crohn’s disease.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Immunity
医学-免疫学
CiteScore
49.40
自引率
2.20%
发文量
205
审稿时长
6 months
期刊介绍:
Immunity is a publication that focuses on publishing significant advancements in research related to immunology. We encourage the submission of studies that offer groundbreaking immunological discoveries, whether at the molecular, cellular, or whole organism level. Topics of interest encompass a wide range, such as cancer, infectious diseases, neuroimmunology, autoimmune diseases, allergies, mucosal immunity, metabolic diseases, and homeostasis.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


