以硼酸、水杨醛和 2-甲酰基吡咯肼为原料,多组分合成立体定向硼荧光团 (BOSPYR)
IF 4.2
2区 化学
Q2 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
这项工作描述了通过多组分反应一步合成各种立体硼荧光染料(BOSPYR),包括容易获得的硼酸,水杨酸醛和2-甲酰吡咯腙。该染料在电磁辐射的可见区吸收和发射,其特点是Stokes位移大(2850-4930 cm−1),荧光发射弱(Φfl: 1.5-9.1%)。值得注意的是,BOSPYRs的暗淡荧光在过渡到粘性介质后恢复(1a为21倍)。具有代表性的化合物1a表现出明显的棉花效应,其不对称因子在可见光区为ca. |gabs| ~ 1.9 × 10−3,表明对发色团的有效不对称诱导。x射线分子结构表明,1a的发色团与平面度偏离17.2°,这可能对荧光团的固有手性有重要影响。利用时变密度泛函理论(TD-DFT)对激发态的计算检验确定了发射机制是由局域激发态引起的。本文章由计算机程序翻译,如有差异,请以英文原文为准。
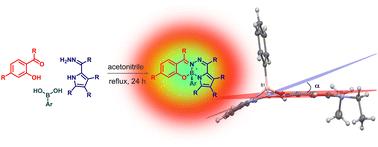
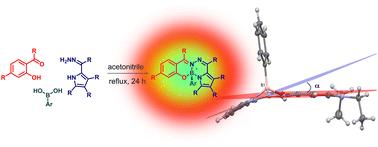
Multicomponent synthesis of stereogenic-at-boron fluorophores (BOSPYR) from boronic acids, salicylaldehydes, and 2-formylpyrrole hydrazones†
This work describes one-step syntheses of various stereogenic-at-boron fluorochromes (BOSPYR) via multicomponent reactions involving readily accessible boronic acids, salicylaldehydes, and 2-formylpyrrole hydrazones. The dyes absorb and emit in the visible region of the electromagnetic radiation, and are characterized by large Stokes shifts (2850–4930 cm−1) with weak fluorescence emissions (Φfl: 1.5–9.1%). Notably, the dimmed fluorescence of BOSPYRs recovers upon transition to viscous media (21-fold for ). The representative compound exhibits clear Cotton effects with dissymmetry factors of ca. |gabs| ∼ 1.9 × 10−3 in the visible region, indicating efficient asymmetry induction to the chromophore. The X-ray molecular structure of shows that the chromophore deviates from planarity by 17.2°, which may contribute significantly to the inherent chirality of the fluorophore. A computational examination of excited states by time-dependent density functional theory (TD-DFT) identifies the emission mechanism as arising from a locally-excited (LE) state.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Chemical Communications
化学-化学综合
CiteScore
8.60
自引率
4.10%
发文量
2705
审稿时长
1.4 months
期刊介绍:
ChemComm (Chemical Communications) is renowned as the fastest publisher of articles providing information on new avenues of research, drawn from all the world''s major areas of chemical research.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


