利用偏振复用技术的折射率形态学成像显微系统,用于无标签单个活细胞
IF 8.2
1区 化学
Q1 CHEMISTRY, ANALYTICAL
引用次数: 0
摘要
本文章由计算机程序翻译,如有差异,请以英文原文为准。
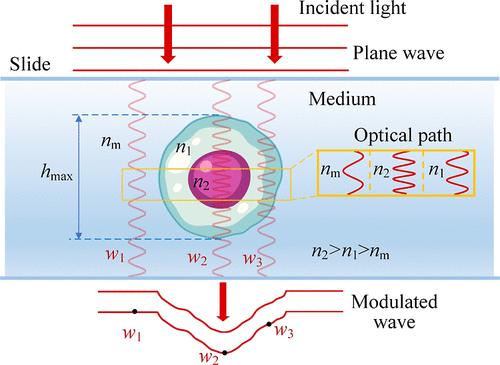
Refractive Index Morphology Imaging Microscope System Utilizing Polarization Multiplexing for Label-Free Single Living Cells
Detections of internal substances and morphologies for label-free living cells are crucial for revealing malignant diseases. With the phase serving as a coupling of refractive index (RI) (marker for substances) and thickness (morphology), existing decoupling methods mainly rely on complex integrated systems or extensive optical field information. Developing simple and rapid decoupling methods remains a challenge. This study introduces a refractive index morphology imaging microscope (RIMIM) system utilizing polarization multiplexing for label-free single living cells. By simultaneous degree of circular polarization (DOCP) imaging and noninterferometric quantitative phase imaging (QPI), the intracellular refractive index distribution (IRID) and morphology can be decoupled. The optical thickness calculated from the phase is input into the circular depolarization decay model (CDDM) of degree of circular polarization to retrieve IRID. Subsequently, the thickness can be decoupled from phase result using retrieved IRID. Experiments conducted on mouse forestomach carcinoma (MFC) cells and human kidney-2 cells (HK-2) demonstrated the RIMIM system’s ability to retrieve IRID and decouple fine morphology. Additionally, the RIMIM system effectively detected membrane damage and changes in erastin-induced ferroptotic HK-2 cells, with average and root-mean-square of surface folds 65.5% and 70.0% higher than those of normal HK-2 cells. Overall, the RIMIM system provides a simple and rapid method for decoupling RI and fine morphology, showing great potential for label-free live cells’ cytopathology detection.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

ACS Sensors
Chemical Engineering-Bioengineering
CiteScore
14.50
自引率
3.40%
发文量
372
期刊介绍:
ACS Sensors is a peer-reviewed research journal that focuses on the dissemination of new and original knowledge in the field of sensor science, particularly those that selectively sense chemical or biological species or processes. The journal covers a broad range of topics, including but not limited to biosensors, chemical sensors, gas sensors, intracellular sensors, single molecule sensors, cell chips, and microfluidic devices. It aims to publish articles that address conceptual advances in sensing technology applicable to various types of analytes or application papers that report on the use of existing sensing concepts in new ways or for new analytes.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


