离子置换诱导的卡戈梅晶格和呼吸卡戈梅晶格的转变
IF 4.7
2区 化学
Q1 CHEMISTRY, INORGANIC & NUCLEAR
引用次数: 0
摘要
通过将PbOCu3(SeO3)2(NO3)F的F -离子取代(OH)−基团合成PbOCu3(SeO3)2(NO3)(OH),显示出kagom本文章由计算机程序翻译,如有差异,请以英文原文为准。
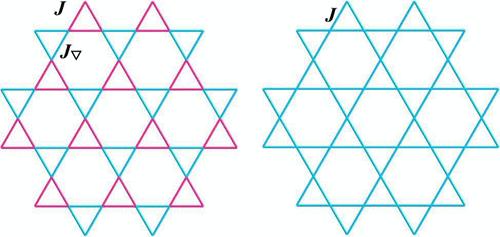
Transformation of Kagomé and Breathing Kagomé Lattices Induced by Ion Replacement
PbOCu3(SeO3)2(NO3)(OH) was synthesized by means of a replacement of (OH)− groups for F– ions of PbOCu3(SeO3)2(NO3)F, showing a transformation of kagomé and breathing kagomé lattices. Such a replacement did not change their intralayer ferromagnetic interactions and interlayer antiferromagnetic (AFM) interactions but slightly affected the Néel temperature and critical field, where PbOCu3(SeO3)2(NO3)(OH) possesses an AFM ordering at TN = 29.3 K, and a field-induced metamagnetic transition can occur at 2 K while a critical magnetic field of 1.45 T is applied.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Inorganic Chemistry
化学-无机化学与核化学
CiteScore
7.60
自引率
13.00%
发文量
1960
审稿时长
1.9 months
期刊介绍:
Inorganic Chemistry publishes fundamental studies in all phases of inorganic chemistry. Coverage includes experimental and theoretical reports on quantitative studies of structure and thermodynamics, kinetics, mechanisms of inorganic reactions, bioinorganic chemistry, and relevant aspects of organometallic chemistry, solid-state phenomena, and chemical bonding theory. Emphasis is placed on the synthesis, structure, thermodynamics, reactivity, spectroscopy, and bonding properties of significant new and known compounds.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


